食管癌是常见的癌症之一,也是全球第六大癌症死因[1]。食管癌的治疗仍以手术为主的肿瘤综合治疗,常伴随较低的生活质量和较高的死亡率,5年生存率约15%~25%[2-3]。微创食管癌切除术(mininally invasiveesophageatomy, MIE)加区域性淋巴结清扫术治疗食管癌于2000年首次报道[4],随着国内外这一技术的不断发展及应用,MIE的安全性和近期疗效已得到国内外学者的认可[5-7]。本研究拟对食管鳞癌行MIE和同期行开放手术患者进行对比,评价近远期疗效,分析影响并发症和预后的因素。
1 资料与方法
1.1 一般资料
选取2014年4月—2016年2月在江苏省盐城市第一人民医院胸外科接受食管癌根治术的术前Ⅰ和Ⅱ期食管鳞癌患者452例,其中行全腔镜食管癌根治术者205例(微创组),行常规手术者247例(开放组)。所有患者术前常规行血常规、生化、凝血功能、肿瘤指标、颈胸腹部增强CT、肺功能、上消化道造影等检查,PET-CT不作为常规检查,明确术前评估和分期,所有患者术前均未行新辅助治疗。
1.2 手术方法及术后处理
根据肿瘤位置,对于食管中上段癌需行颈部吻合的患者采用传统颈胸腹三切口食管癌根治术(McKeown术),对于食管中下段癌患者常采用右胸腹两切口食管癌根治术(Ivor-Lewis术)。微创组包括全腔镜McKeown术和全腔镜Ivor-Lewis术。所有患者术前6 h常规进食牛奶或肠内营养液,以便于术中显露胸导管,全腔镜McKeown术采用全身麻醉后单腔气管插管,取侧俯卧位,建立人工气胸,胸腔镜下完成食管游离,清扫食管旁、左右喉返神经旁、隆突下、肺门旁、膈上、胸导管旁等淋巴结;改平卧位后,建立人工气腹,腹腔镜下游离胃制成管状胃,清扫腹腔区域淋巴结;左颈部切口行食管胃侧侧吻合。术后常规留置胃管和十二指肠营养管,术后第1天开始胃管留置状态下2 h进食清水1次,同时常规肠内营养支持。全腔镜Ivor-Lewis术采用全身麻醉后双腔气管插管,取平卧位,人工气腹下采用五孔法完成胃的游离,见图1。腹腔镜下使用血管夹离断小弯侧血管,见图2。使用腔镜下直线切割缝合器沿胃小弯侧裁剪制作管状胃,见图3。清扫腹腔区域淋巴结,改左侧卧位后,三孔法完成食管游离及周围淋巴结清扫,胸腔镜下行食管胃“T型”吻合,术后留置胃管,术后第1天开始留置胃管状态下2 h进食清水1次,同时给予肠外营养支持,术后肠道通气后可拔除胃管。术后采用疼痛面部表情分级评分法(Face Rating Scale, FRS)评价患者术后疼痛,满分10分,分值越大,疼痛程度越高,对于评分大于6分(严重疼痛)患者常规给予阿片类镇痛剂;术后双侧肺呼吸音好,胸瓶内无气体溢出,引流液颜色无浑浊或乳糜色,24 h量少于100 mL,胸片示肺复张良好,可拔除胸管。患者进食半流质无特殊不适,无发热、胸闷气喘等症状,复查血生化、消化道造影等检查无异常,可予出院。记录两组间手术时间、术中出血量、胸腔引流时间、术后住院时间、麻醉镇痛剂使用数、淋巴结数等。对于pT2-4N1-2M0可耐受治疗的患者术后行辅助放、化疗[8-9]。

图1 微创Ivor-Lewis腹部操作孔
Fig.1 Abdominal operation hole of minimally invasive Ivor-Lewis
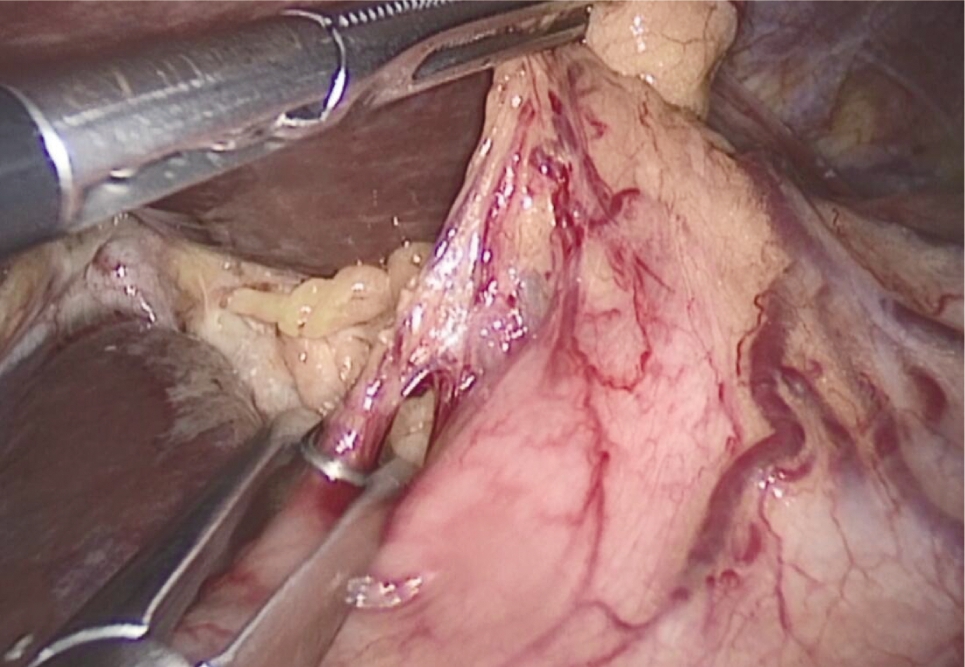
图2 离断胃小弯血管
Fig.2 Detach lesser gastric curvature
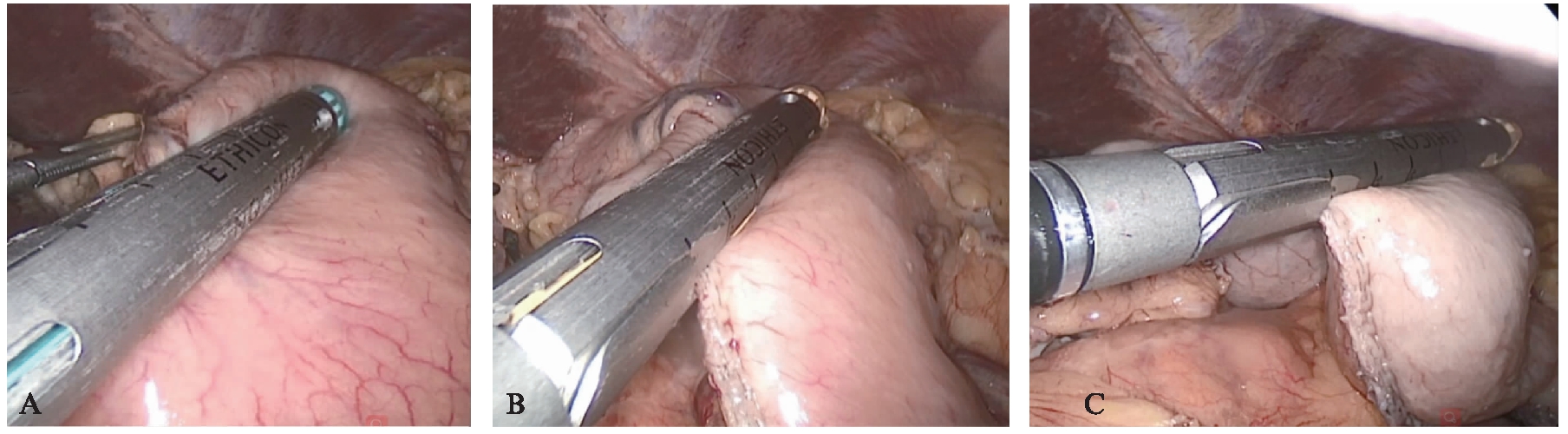
图3 腹腔镜下制作管状胃
Fig.3 Construct gastric conduit by laparoscopy
A:沿胃小弯制作管状胃;B:向胃底裁前管胃;C:离断管胃与胃底
1.3 随访
术后通过电话、门诊复查、微信等方式随访,前2年每3个月随访1次,后每半年随访1次,内容包括症状、体征、实验室检查、复发、生存等情况;总生存期定义为从手术至死亡的时间。随访截止时间为2020年10月或患者死亡。
1.4 统计学处理
所有统计资料采用SPSS 23.0软件分析完成。计数资料用百分比表示,组间比较采用χ2检验。计量资料符合正态分布的采用![]() 表示,组间均数比较用独立样本t检验。多因素Logistic回归模型分析影响术后并发症的因素。单因素生存分析采用Kaplan-Meier法,差异性比较采用Log-rank检验,多因素生存分析采用Cox风险比例模型来分析影响预后的因素。P<0.05为差异有统计学意义。
表示,组间均数比较用独立样本t检验。多因素Logistic回归模型分析影响术后并发症的因素。单因素生存分析采用Kaplan-Meier法,差异性比较采用Log-rank检验,多因素生存分析采用Cox风险比例模型来分析影响预后的因素。P<0.05为差异有统计学意义。
2 结 果
2.1 两组基本资料比较
开放组和微创组在性别、年龄、ASA分级、TNM分期、分化程度、术者例数、肿瘤位置、术前FEV1/FVC(%)、体质量指数(body massindex, BMI)、鳞状细胞癌抗原(squamous cell carcinoma antigen, SCCA)、血红蛋白(hemoglobin, Hb)等方面差异无统计学意义(P>0.05),而亚组微创McKeown术组多于开放组、微创Ivor-Lewis术组少于开放组,差异有统计学意义(P<0.05),见表1。
表1 两组患者基本资料比较
Tab.1 Comparison of basic data between the two groups
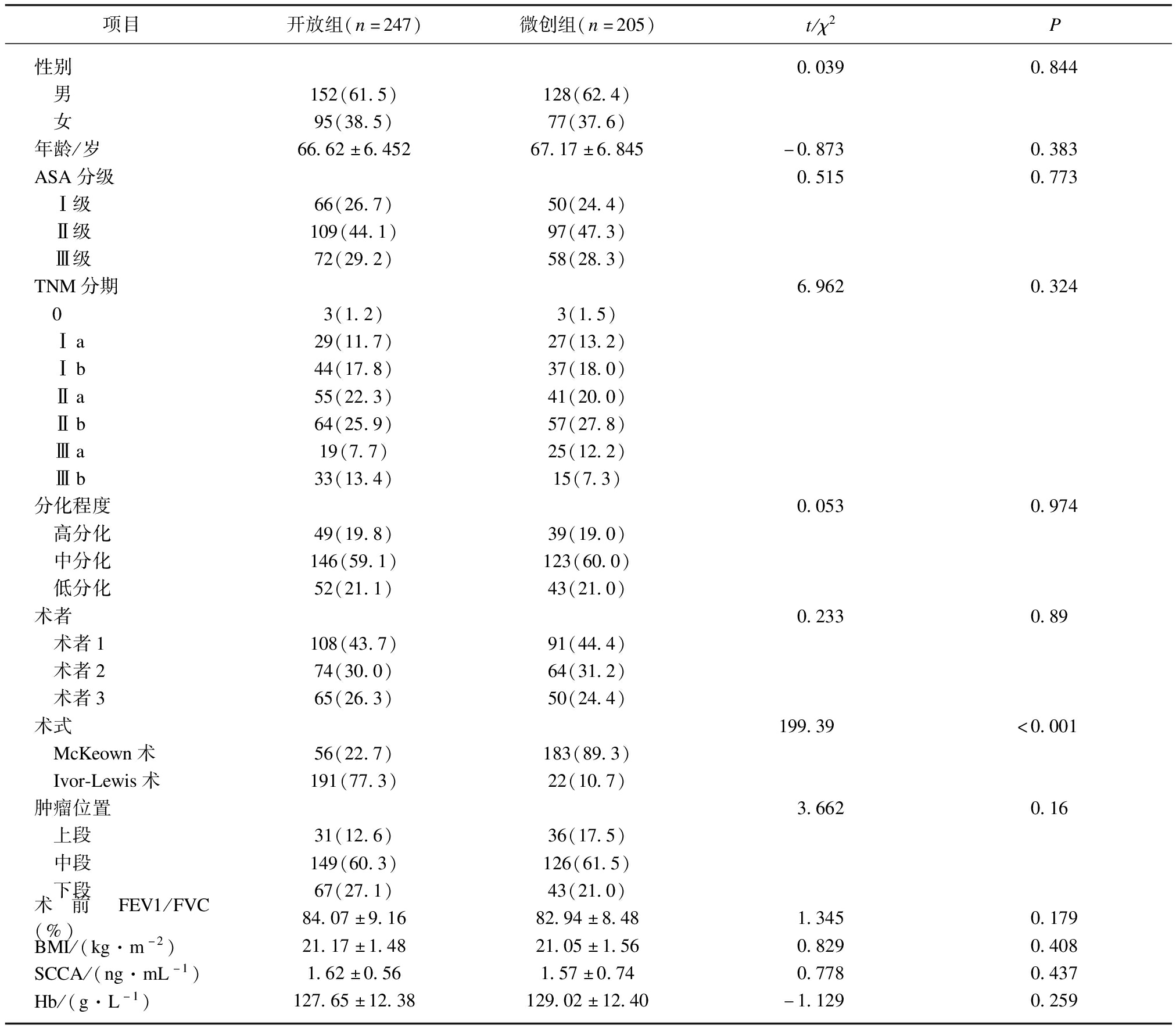
项目开放组(n=247)微创组(n=205)t/χ2P性别0.0390.844 男152(61.5)128(62.4) 女95(38.5)77(37.6)年龄/岁66.62±6.45267.17±6.845-0.8730.383ASA分级0.5150.773 Ⅰ级66(26.7)50(24.4) Ⅱ级109(44.1)97(47.3) Ⅲ级72(29.2)58(28.3)TNM分期6.9620.324 03(1.2)3(1.5) Ⅰa29(11.7)27(13.2) Ⅰb44(17.8)37(18.0) Ⅱa55(22.3)41(20.0) Ⅱb64(25.9)57(27.8) Ⅲa19(7.7)25(12.2) Ⅲb33(13.4)15(7.3)分化程度0.0530.974 高分化49(19.8)39(19.0) 中分化146(59.1)123(60.0) 低分化52(21.1)43(21.0)术者0.2330.89 术者1108(43.7)91(44.4) 术者274(30.0)64(31.2) 术者365(26.3)50(24.4)术式199.39<0.001 McKeown术56(22.7)183(89.3) Ivor-Lewis术191(77.3)22(10.7)肿瘤位置3.6620.16 上段31(12.6)36(17.5) 中段149(60.3)126(61.5) 下段67(27.1)43(21.0)术前FEV1/FVC(%)84.07±9.1682.94±8.481.3450.179BMI/(kg·m-2)21.17±1.4821.05±1.560.8290.408SCCA/(ng·mL-1)1.62±0.561.57±0.740.7780.437Hb/(g·L-1)127.65±12.38129.02±12.40-1.1290.259
ASA:美国麻醉师协会,SCCA:鳞状细胞癌抗原,Hb:血红蛋白
2.2 两组围手术期资料比较
两组患者围手术期资料比较见表2,微创组术中出血量、胸腔引流时间、术后住院时间、麻醉镇痛剂使用数少于开放组,差异有统计学意义(P<0.05);两组R0切除率、双侧喉返神经旁淋巴结清扫成功率、左右喉返神经旁淋巴结清扫数、总体淋巴结清扫数相当,差异无统计学意义。亚组分析显示,微创McKeown术手术时间与开放组相当,微创Ivor-Lewis手术时间长于开放组。微创组有9例中转开胸或开腹,中转率4.2%(9/214),考虑对结果影响未入组。全组无围手术期死亡病例,术后有并发症患者多在术后30 d内康复,未影响进一步肿瘤综合治疗。
表2 两组患者围手术期资料比较
Tab.2 Comparison of perioperative data between the two groups![]()
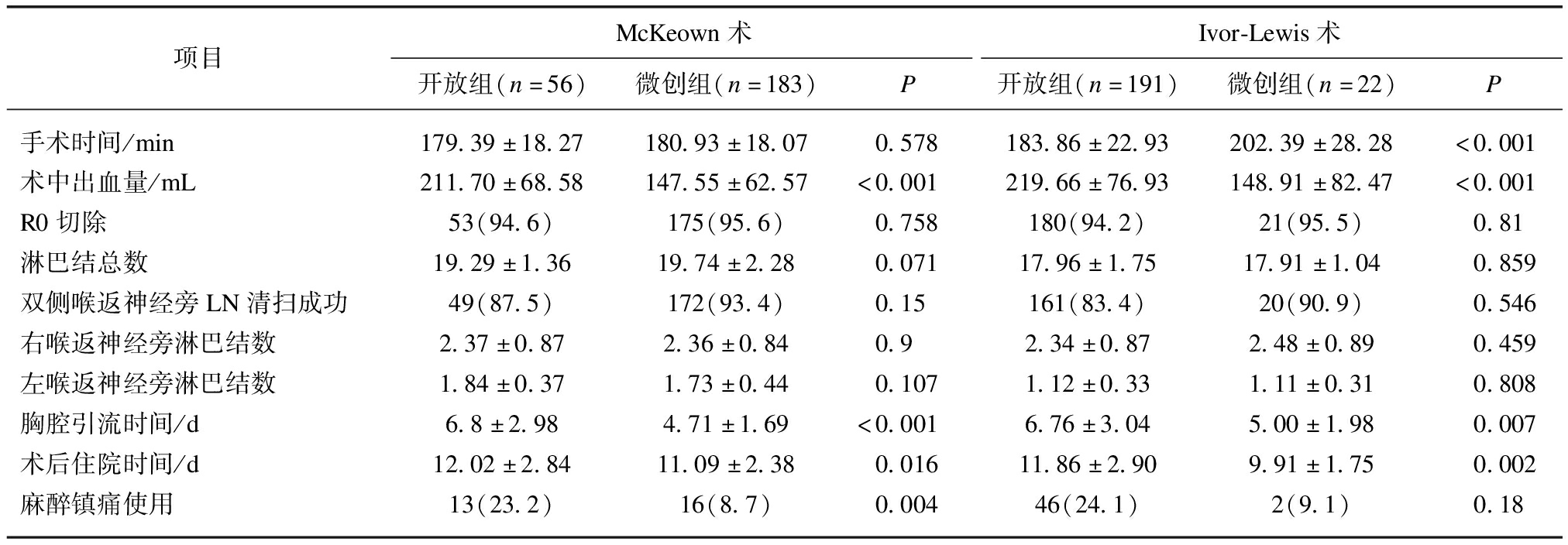
项目McKeown术Ivor-Lewis术开放组(n=56)微创组(n=183)P开放组(n=191)微创组(n=22)P手术时间/min179.39±18.27180.93±18.070.578183.86±22.93202.39±28.28<0.001术中出血量/mL211.70±68.58147.55±62.57<0.001219.66±76.93148.91±82.47<0.001R0切除53(94.6)175(95.6)0.758180(94.2)21(95.5)0.81淋巴结总数19.29±1.3619.74±2.280.07117.96±1.7517.91±1.040.859双侧喉返神经旁LN清扫成功49(87.5)172(93.4)0.15161(83.4)20(90.9)0.546右喉返神经旁淋巴结数2.37±0.872.36±0.840.92.34±0.872.48±0.890.459左喉返神经旁淋巴结数1.84±0.371.73±0.440.1071.12±0.331.11±0.310.808胸腔引流时间/d6.8±2.984.71±1.69<0.0016.76±3.045.00±1.980.007术后住院时间/d12.02±2.8411.09±2.380.01611.86±2.909.91±1.750.002麻醉镇痛使用13(23.2)16(8.7)0.00446(24.1)2(9.1)0.18
2.3 两组术后并发症比较
微创组术后并发症数低于开放组,多因素Logistic回归模型分析影响术后并发症的因素,结果显示,TNM分期、ASA、不同术者、手术方式、FEV1/FVC是影响术后并发症的相关因素,见表3。TNM分期越晚、FEV1/FVC下降、ASA越大、术者手术量越小是影响术后并发症的不利因素(P<0.05),而手术方式(微创组)是影响术后并发症的有利因素(P<0.05),见表4。
表3 两组患者术后并发症比较
Tab.3 Comparison of postoperative complications between the two groups
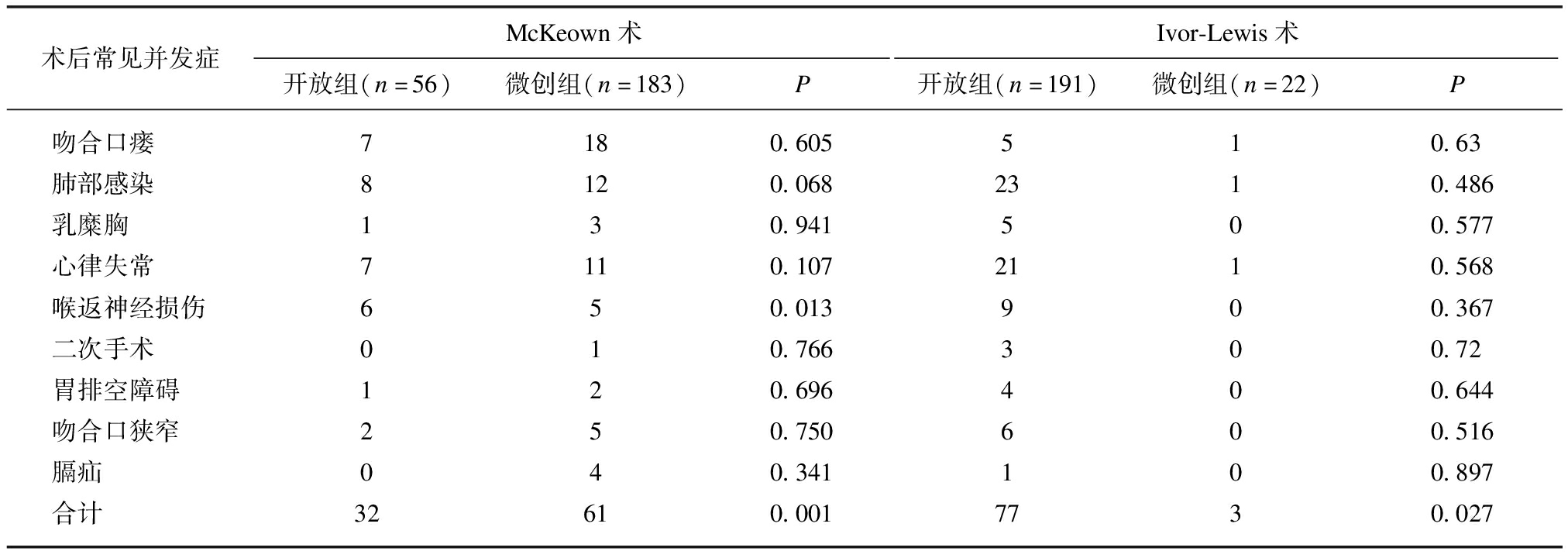
术后常见并发症McKeown术Ivor-Lewis术开放组(n=56)微创组(n=183)P开放组(n=191)微创组(n=22)P吻合口瘘7180.605510.63肺部感染8120.0682310.486乳糜胸130.941500.577心律失常7110.1072110.568喉返神经损伤650.013900.367二次手术010.766300.72胃排空障碍120.696400.644吻合口狭窄250.750600.516膈疝040.341100.897合计32610.0017730.027
表4 影响术后并发症的多因素Logistic回归分析结果
Tab.4 Multivariate Logistic regression analysis of postoperative complications
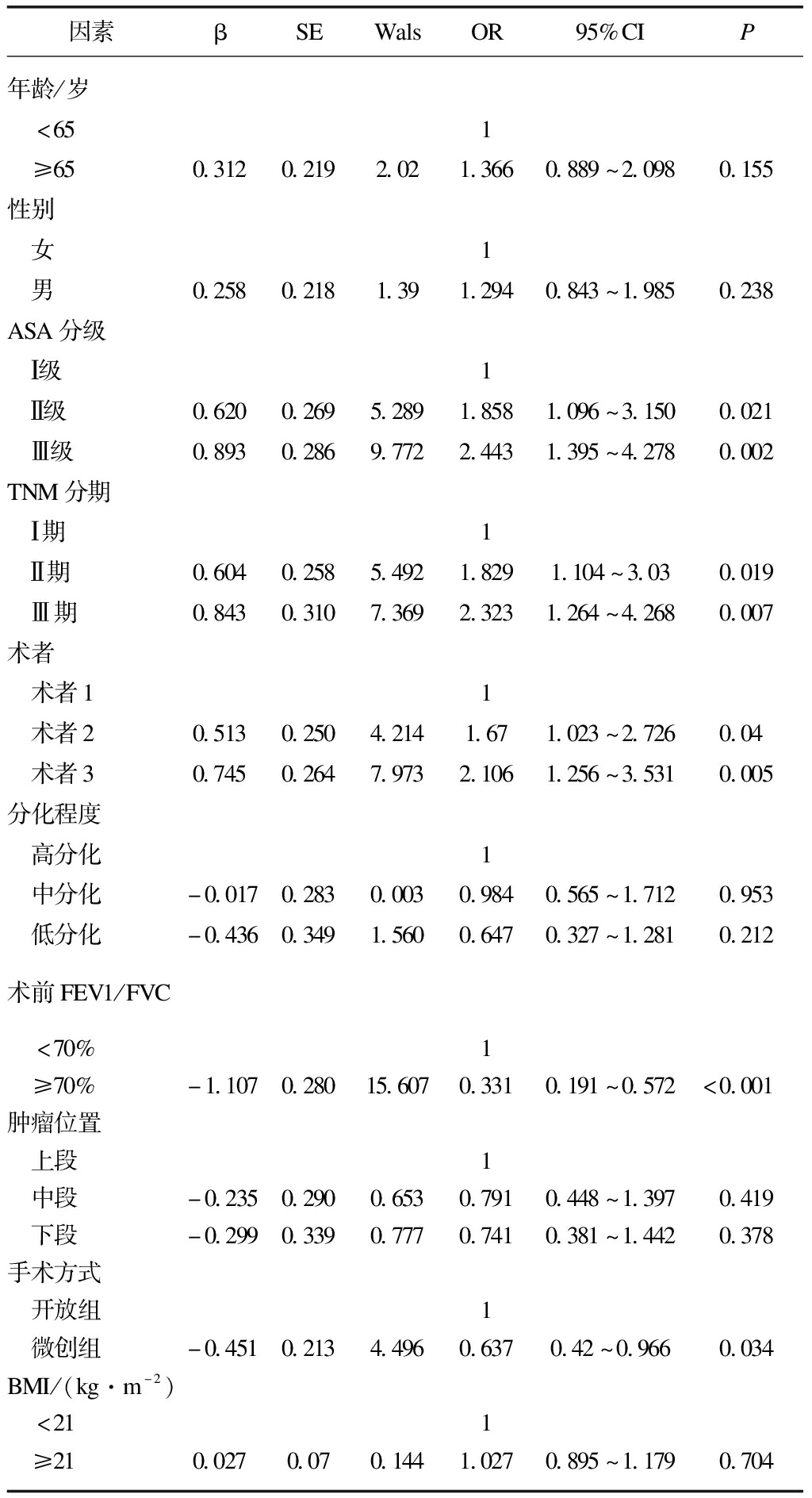
因素βSEWalsOR95%CIP年龄/岁 <651 ≥650.3120.2192.021.3660.889~2.0980.155性别 女1 男0.2580.2181.391.2940.843~1.9850.238ASA分级 Ⅰ级1 Ⅱ级0.6200.2695.2891.8581.096~3.1500.021 Ⅲ级0.8930.2869.7722.4431.395~4.2780.002TNM分期 Ⅰ期1 Ⅱ期0.6040.2585.4921.8291.104~3.030.019 Ⅲ期0.8430.3107.3692.3231.264~4.2680.007术者 术者11 术者20.5130.2504.2141.671.023~2.7260.04 术者30.7450.2647.9732.1061.256~3.5310.005分化程度 高分化1 中分化-0.0170.2830.0030.9840.565~1.7120.953 低分化-0.4360.3491.5600.6470.327~1.2810.212术前FEV1/FVC <70%1 ≥70%-1.1070.28015.6070.3310.191~0.572<0.001肿瘤位置 上段1 中段-0.2350.2900.6530.7910.448~1.3970.419 下段-0.2990.3390.7770.7410.381~1.4420.378手术方式 开放组1 微创组-0.4510.2134.4960.6370.42~0.9660.034BMI/(kg·m-2) <211 ≥210.0270.070.1441.0270.895~1.1790.704
2.4 随访结果
本组共失访27例,最终纳入452例,随访率94.3%。开放组中位生存时间为(38±2.6)个月,95%置信区间为32.87~43.13个月;微创组中位生存时间为(45±7.8)个月,95%置信区间为29.68~60.32个月。开放组1、3、5年总体生存率为85.8%,53.4%,39.2%;微创组1、3、5年总体生存率为86.3%、58.5%、44.4%,微创组远期生存略优于开放组,差异无统计学意义(P>0.05),见图4及表5、6。

图4 微创组和开放组两组总体生存率曲线图
Fig.4 Curves for comparison of overall survival between minimally invasive and open esophagectomy groups
2.5 单因素方差分析
单因素分析显示年龄、ASA分级、TNM分期、分化程度、不同术者、肿瘤位置、鳞状细胞癌抗原、血红蛋白是影响远期生存的因素(P<0.05),见表5。
表5 Kaplan-Meier单因素分析结果
Tab.5 Results of Kaplan-Meier univariate analysis
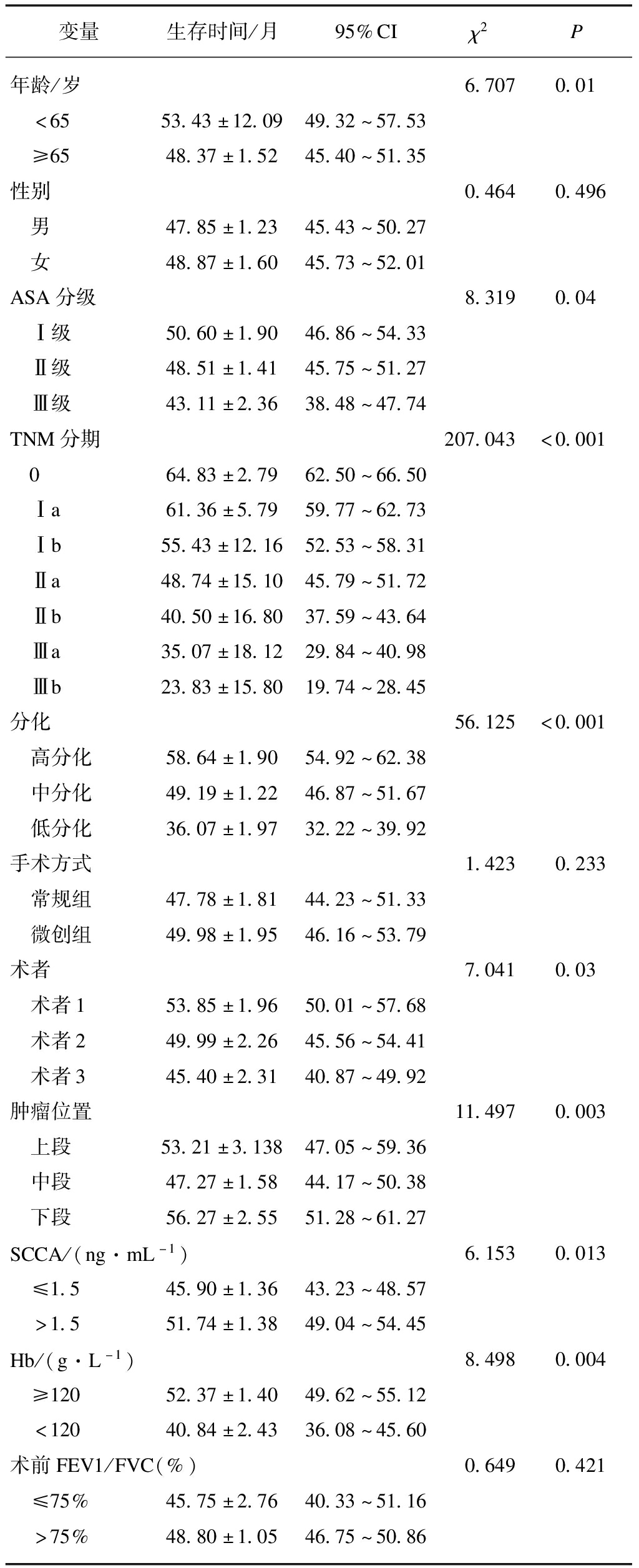
变量生存时间/月95%CIχ2P年龄/岁 6.7070.01 <6553.43±12.0949.32~57.53 ≥6548.37±1.5245.40~51.35性别0.4640.496 男47.85±1.2345.43~50.27 女48.87±1.6045.73~52.01ASA分级8.3190.04 Ⅰ级50.60±1.9046.86~54.33 Ⅱ级48.51±1.4145.75~51.27 Ⅲ级43.11±2.3638.48~47.74TNM分期207.043<0.001 064.83±2.7962.50~66.50 Ⅰa61.36±5.7959.77~62.73 Ⅰb55.43±12.1652.53~58.31 Ⅱa48.74±15.1045.79~51.72 Ⅱb40.50±16.8037.59~43.64 Ⅲa35.07±18.1229.84~40.98 Ⅲb23.83±15.8019.74~28.45分化56.125<0.001 高分化58.64±1.9054.92~62.38 中分化49.19±1.2246.87~51.67 低分化36.07±1.9732.22~39.92手术方式1.4230.233 常规组47.78±1.8144.23~51.33 微创组49.98±1.9546.16~53.79术者7.0410.03 术者153.85±1.9650.01~57.68 术者249.99±2.2645.56~54.41 术者345.40±2.3140.87~49.92肿瘤位置11.4970.003 上段53.21±3.13847.05~59.36 中段47.27±1.5844.17~50.38 下段56.27±2.5551.28~61.27SCCA/(ng·mL-1)6.1530.013 ≤1.545.90±1.3643.23~48.57 >1.551.74±1.3849.04~54.45Hb/(g·L-1)8.4980.004 ≥12052.37±1.4049.62~55.12 <12040.84±2.4336.08~45.60术前FEV1/FVC(%)0.6490.421 ≤75%45.75±2.7640.33~51.16 >75%48.80±1.0546.75~50.86
2.6 多因素分析
多因素Cox风险模型调整了年龄、性别、分期、ASA分级、术者、肿瘤位置、血红蛋白等相关混杂因素后,分析结果显示年龄、TNM分期、分化程度是影响预后的独立因素,见表6。
表6 Cox多因素风险比例模型总体生存分析结果
Tab.6 Multivariable Cox proportional hazard regression for overall survival
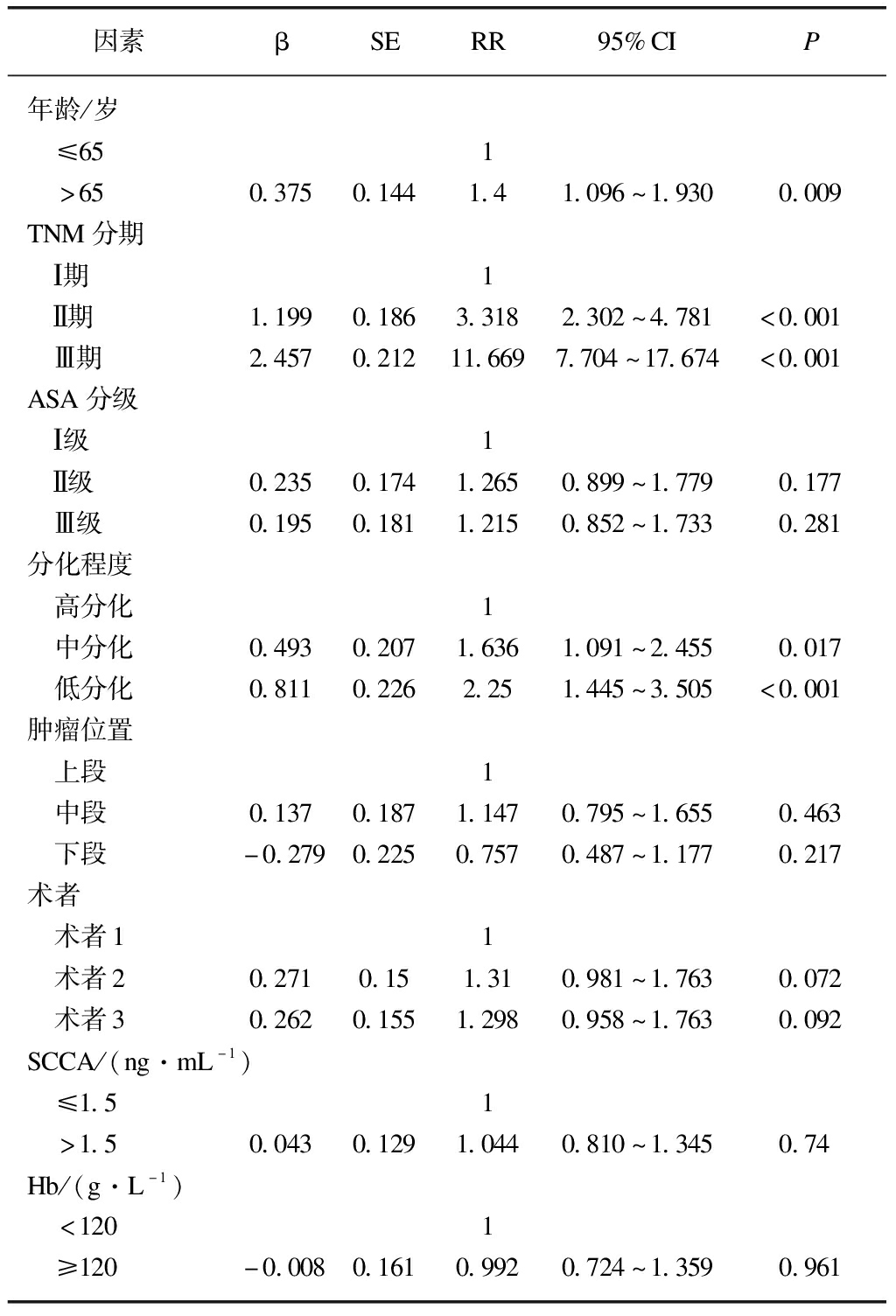
因素βSERR95%CIP年龄/岁 ≤651 >650.3750.1441.41.096~1.9300.009TNM分期 Ⅰ期1 Ⅱ期1.1990.1863.3182.302~4.781<0.001 Ⅲ期2.4570.21211.6697.704~17.674<0.001ASA分级 Ⅰ级1 Ⅱ级0.2350.1741.2650.899~1.7790.177 Ⅲ级0.1950.1811.2150.852~1.7330.281分化程度 高分化1 中分化0.4930.2071.6361.091~2.4550.017 低分化0.8110.2262.251.445~3.505<0.001肿瘤位置 上段1 中段0.1370.1871.1470.795~1.6550.463 下段-0.2790.2250.7570.487~1.1770.217术者 术者11 术者20.2710.151.310.981~1.7630.072 术者30.2620.1551.2980.958~1.7630.092SCCA/(ng·mL-1) ≤1.51 >1.50.0430.1291.0440.810~1.3450.74Hb/(g·L-1) <1201 ≥120-0.0080.1610.9920.724~1.3590.961
3 讨 论
近年来,由于开放手术治疗食管癌的创伤大,术后并发症和死亡率高,随着微创技术的日趋成熟,微创食管癌根治术已逐步应用于临床治疗食管癌,且已证实微创手术较开放手术可降低术后疼痛、减少术后肺部并发症、缩短住院时间等优势[5,10]。但也有结果显示MIE术后并发症和围手术期死亡率与开放手术无明显差异[11]。本研究比较了205例全腔镜食管癌根治术和247例常规开放手术治疗食管癌的围手术期资料,分析其疗效,探讨影响并发症的因素,评价其远期疗效,分析影响预后的因素。
本组结果显示,微创组术中出血量、胸腔引流时间、术后住院时间、麻醉镇痛剂使用数等方面优于开放手术组,与文献报道一致[5,10]。微创McKeown组手术时间与开放组相当,而微创Ivor-Lewis组手术时间长于开放组,主要是腔镜开展初期,有一定的学习曲线,手术时间相对较长,尤其腔镜下胸内食管胃吻合操作难度高,随着后期技术的成熟,微创组手术时间少于开放组,主要因腔镜减少了开关胸腹切口的时间,且对于部分肥胖、胸腔粘连等患者腔镜下操作更具优势。本组患者常规进行“两野”淋巴结清扫,两组淋巴结清扫总数相当,且两组左右喉返神经旁淋巴结清扫数和清扫成功率无差异,说明微创组可达到与开放组同样的淋巴结清扫效果。也有文献[6,10,12]表明MIE清扫淋巴结优于常规组,主要因人工气胸下腔镜可以更好地显露纵隔,可以更清晰的解剖显露左右喉返神经,便于清扫左右喉返神经旁淋巴结;腹腔镜下可以更好实现腹腔血管“骨骼化”及打开筋膜间隙,显露腹腔深部,更好地清扫腹腔深部的第7~11站淋巴结。
评价一种术式的安全性和优越性主要在于术中的操作安全性和术后的并发症发生率,微创组术后总体并发症数少于开放组,与相关研究结果一致[6,13]。吻合口瘘仍是食管癌术后最常见的并发症之一[14],本组微创术后吻合口瘘略多于开放手术组,但差异无统计学意义,主要由于微创组多采用颈部吻合,与全腔镜McKeown术颈部吻合口血供相对差、张力大,以及术者吻合技巧等因素有关[15],而全腔镜Ivor-Lewis术后吻合瘘与开放手术相当,说明腔镜下胸内吻合可达到与开放相当的安全性。与应开军等[16]的报道相比,本研究认为微创术后早期进食清水等流质,可湿润清洁咽喉部,利于咳嗽排痰,减少肺部感染,且并未增加吻合口瘘的发生。之前的结果显示MIE术后较常规Ivor-Lewis术可增加膈疝风险,主要与微创手术减少腹腔粘连、增大了食管裂孔、胃结肠韧带游离不充分等原因有关[17]。微创组较开放组可降低术后肺部感染、心律失常并发症,主要与微创疼痛轻,术中减少对肺牵拉等副损伤,保持了胸廓完整性,术后患者可以有效咳嗽排痰,减少肺不张,且微创创伤小,降低了炎性介质对心肺血管的刺激。研究表明手术量多的术者行食管癌术后30、90 d内死亡率显著降低[18],本研究结果也显示不同术者是影响术后并发症的因素,主要与不同术者的手术量、手术方式选择、解剖辨认、操作技巧等因素有关。TNM分期、术前肺功能、ASA分级也是影响术后并发症的因素,因此,为了减少术后并发症的发生,可术前锻炼改善肺功能,积极处理合并症,不断提高术者的手术操作技巧及手术量,选择合理的个体化手术方式,对于肿瘤分期较晚的患者可术前新辅助治疗以达到肿瘤降期。
手术治疗肿瘤的疗效评价主要是术后远期生存率。一项Meta分析证实了微创手术治疗食管鳞癌远期疗效优于开放组[19],本研究的结果也显示,微创术后总体生存率略优于开放组,微创手术可以达到与开放手术组同样的远期疗效[6,20]。微创手术创伤小,术后恢复快,术后可以保护自身固有免疫以减少肿瘤复发[19],微创术后较开放手术患者可更好地耐受术后放化疗,而食管癌术后放疗可提高远期生存,且微创术后并发症低于开放手术组,而术后并发症与远期生存率低有关[21]。
本研究为单中心回顾性分析,不排除受样本量小、选择性偏移等因素影响,与相关报道[19]相反,对于年龄大、合并症多、肺功能差的患者更倾向选择腔镜手术,因此本研究纳入了不同术者、ASA分级等因素进行多因素分析减少混淆误差。本组单因素分析年龄、ASA分级、TNM分期、分化程度、不同术者、肿瘤位置、鳞状细胞癌抗原、血红蛋白是影响预后的因素,而Cox多因素分析显示年龄、TNM分期、分化程度是影响预后的独立因素,说明微创手术不是影响预后的独立因素,微创可以达到或优于开放手术的远期疗效。多因素分析SCCA、Hb不是影响预后的独立因素,主要因SCCA增高、Hb降低多提示患者分期较晚。研究证实,不同术者的累计手术量与远期预后相关,手术量多的术者行食管癌术后患者可获得更好的远期生存[22]。本研究单因素分析结果也显示累计手术量少的术者行手术后患者的预后略差于累计手术量多的术者,但不同术者不是影响预后的独立因素,主要受样本量少及术者对不同术式、手术适应证的选择等因素影响。年龄越大,合并症越多,ASA分级相对越大,术后并发症相对较多,且对术后辅助治疗的耐受更差,远期生存较差。
总之,微创手术治疗食管癌较开放手术可减轻术后疼痛、缩短住院时间和降低术后并发症,且可达到等同于甚至优于常规手术的远期疗效。提高微创手术经验和手术量,选择个体化治疗方案,也利于减少术后并发症和提高远期生存。
[1] GLOBAL BURDEN OF DISEASE CANCER COLLABORATION, FITZMAURICE C, DICKER D, et al.The global burden of cancer 2013[J].JAMA Oncol, 2015,1(4):505-527.
[2] JACOBS M, MACEFIELD R C, ELBERS R G, et al.Meta-analysis shows clinically relevant and long-lasting deterioration in health-related quality of life after esophageal cancer surgery[J].Qual Life Res, 2014,23(4):1155-1176.
[3] GUPTA B, KUMAR N.Worldwide incidence, mortality and time trends for cancer of the oesophagus[J].Eur J Cancer Prev, 2017,26(2):107-118.
[4] LUKETICH J D, SCHANER P R, CHRISTIE N A, et al.Minimally invasive esophagectomy[J].Ann Thorac Surg, 2000,70(3):906-912.
[5] BIERE S S, VAN BERGE HENEGOUWEN M I, MAAS K W, et al.Minimally invasive versus open oesophagectomy for patients with oesophageal cancer:a multicentre, open-label, randomised controlled trial[J].Lancet, 2012,379(9829):1887-1892.
[6] 孙超,石维平,吕小夏,等.三切口食管癌切除术:胸腔镜与开放手术的术后并发症分析[J].中华腔镜外科杂志(电子版),2016,9(6):354-357.
[7] 张彤,马永富,刘阳.微创食管切除术治疗食管癌的研究进展[J].中华腔镜外科杂志(电子版),2020,13(1):57-60.
[8] YU S F, ZHANG W C, NI W J, et al.A propensity-score matching analysis comparing long-term survival of surgery alone and postoperative treatment for patients in node positive or stage Ⅲ esophageal squamous cell carcinoma after R0 esophagectomy[J].Radiother Oncol, 2019,140:159-166.
[9] KANG J J, CHANG J Y, SUN X, et al.Role of postoperative concurrent chemoradiotherapy for esophageal carcinoma:a meta-analysis of 2 165 patients[J].J Cancer, 2018,9(3):584-593.
[10] WANG K X, ZHONG J, LIU Q W, et al.A propensity score-matched analysis of thoracolaparoscopic vs open McKeown’s esophagectomy[J].Ann Thorac Surg, 2021:S0003-S4975(21)00307-6.
[11] MAMIDANNA R, BOTTLE A, AYLIN P, et al.Short-term outcomes following open versus minimally invasive esophagectomy for cancer in England[J].Ann Surg, 2012,255(2):197-203.
[12] CHEN L, LIU X, WANG R, et al.Minimally invasive esophagectomy for esophageal cancer according to the location of the tumor:experience of 251 patients[J].Ann Med Surg, 2017,17:54-60.
[13] YIBULAYIN W, ABULIZI S, LV H, et al.Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer:a meta-analysis[J].World J Surg Oncol, 2016,14(1):304.
[14] LOW D E, ALDERSON D, CECCONELLO I, et al.International consensus on standardization of data collection for complications associated with esophagectomy[J].Ann Surg, 2015,262(2):286-294.
[15] VAN WORKUM F, VAN DER MAAS J, VAN DEN WILDENBERG F J, et al.Improved functional results after minimally invasive esophagectomy:intrathoracic versus cervical anastomosis[J].Ann Thorac Surg, 2017,103(1):267-273.
[16] 应开军,王大泉,孙云浩,等.全腔镜下Ivor-Lewis术与McKeown术治疗食管癌的临床疗效[J].局部手术解剖学,2021,30(4):321-324.
[17] 孙云浩,应开军,刘国锋,等.微创与常规Ivor-Lewis食管癌手术后膈疝的比较及诊治[J].中华胸心血管外科杂志,2018,34(6):376-378.
[18] MARKAR S R, MACKENZIE H, ASKARI A, et al.Effect of esophageal cancer surgeon volume on management and mortality from emergency upper gastrointestinal conditions:population-based cohort study[J].Ann Surg, 2017,266(5):847-853.
[19] GOTTLIEB-VEDI E, KAUPPILA J H, MALIETZIS G, et al.Long-term survival in esophageal cancer after minimally invasive compared to open esophagectomy:a systematic review and meta-analysis[J].Ann Surg, 2019,270(6):1005-1017.
[20] STRAATMAN J, VAN DER WIELEN N, CUESTA M A, et al.Minimally invasive versus open esophageal resection:three-year follow-up of the previously reported randomized controlled trial:the TIME trial[J].Ann Surg, 2017,266(2):232-236.
[21] AAHLIN E K, OLSEN F, ULEBERG B, et al.Major postoperative complications are associated with impaired long-term survival after gastro-esophageal and pancreatic cancer surgery:a complete national cohort study[J].BMC Surg, 2016,16(1):1-8.
[22] DEROGAR M, SADR-AZODI O, JOHAR A, et al.Hospital and surgeon volume in relation to survival after esophageal cancer surgery in a population-based study[J].J Clin Oncol, 2013,31(5):551-557.