急性肺损伤(acute lung injury, ALI)是由多种原因引起的以急性、进行性呼吸功能不全为特征的呼吸系统危重疾病,急性肺损伤进一步可发展为急性呼吸窘迫综合征(acute respiratory distress syndrome, ARDS)。研究发现ARDS的患病率和死亡率非常高,重度ARDS死亡率可高达46.1%[1]。急性肺损伤主要损伤了肺泡上皮细胞、血管内皮细胞及巨噬细胞,导致细胞形态、结构和功能发生改变,因此,从细胞和分子病理学层面探索新的治疗方法和治疗药物改善细胞损伤成为当前热点课题,由于微小RNA(microRNA, miRNA)在许多疾病中特异性表达(显著上调或下调),并在患者血清中容易检测且灵敏度高,因此可作为研究的重要切入点。
1 miRNA的基本分子特征
miRNA在细胞核内合成,主要被RNA聚合酶Ⅱ转录为初始miRNA,然后被Drosha/DGCR8复合体剪切成70~100个核苷酸的前体miRNA,再通过输出蛋白5转移到细胞质被Dicer/TRBP复合体处理为21~25个核苷酸的双链RNA分子,最后降解成单链的成熟miRNA与特定靶基因的3’-UTR(非翻译区)结合,降解mRNA或抑制其蛋白质翻译[2]。目前已通过高通量测序、基因芯片等技术筛选出大量与肺损伤相关的miRNAs,并在动物及细胞等层面验证miRNAs参与了肺损伤的调控见表1~2。
表1 miRNAs在ALI中表达谱变化
Tab.1 Changes of expression profile miRNAs in ALI
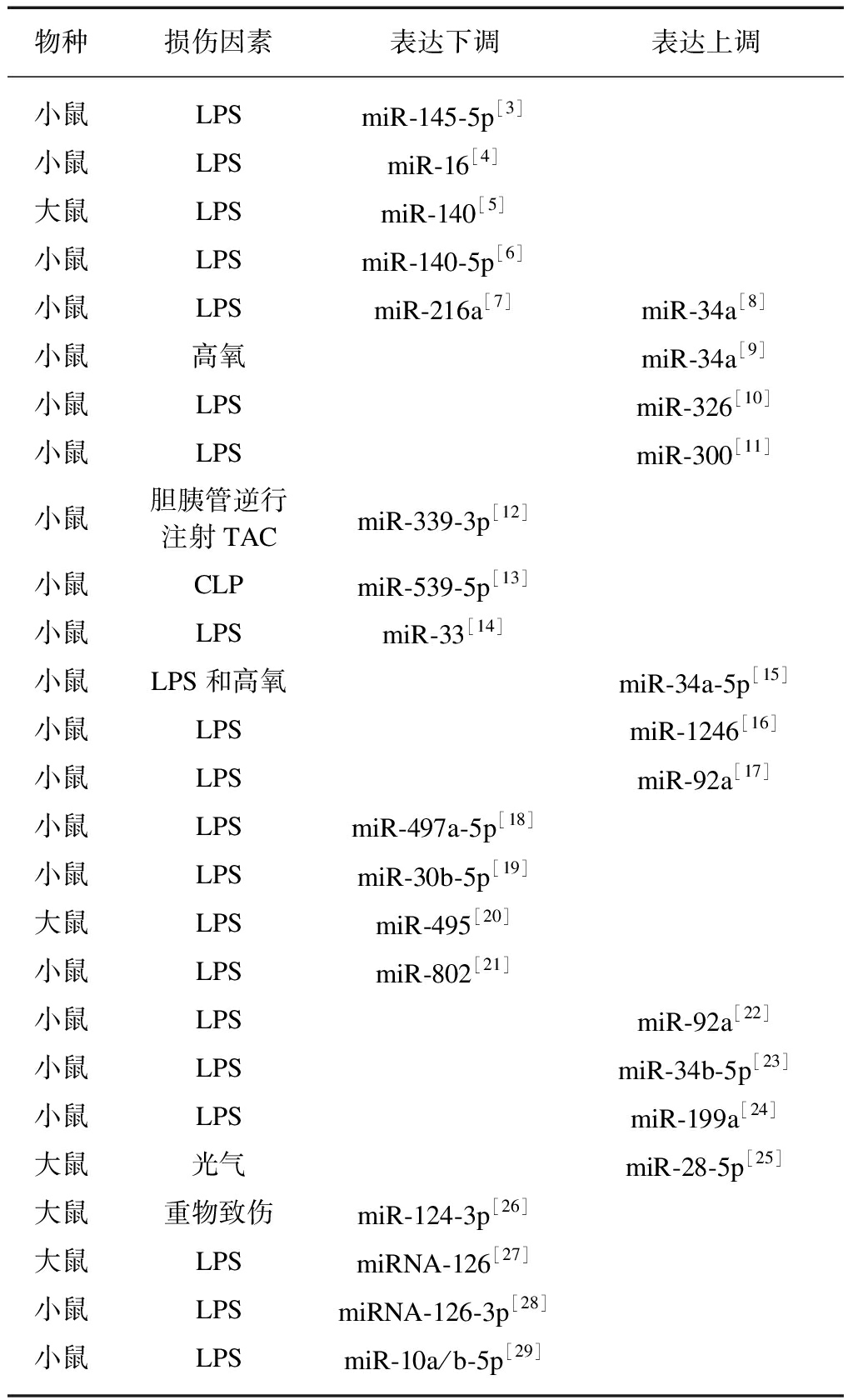
物种损伤因素表达下调表达上调小鼠LPSmiR-145-5p[3]小鼠LPSmiR-16[4]大鼠LPSmiR-140[5]小鼠LPSmiR-140-5p[6]小鼠LPSmiR-216a[7]miR-34a[8]小鼠高氧miR-34a[9]小鼠LPSmiR-326[10]小鼠LPSmiR-300[11]小鼠胆胰管逆行注射TACmiR-339-3p[12]小鼠CLPmiR-539-5p[13]小鼠LPSmiR-33[14]小鼠LPS和高氧miR-34a-5p[15]小鼠LPSmiR-1246[16]小鼠LPSmiR-92a[17]小鼠LPSmiR-497a-5p[18]小鼠LPSmiR-30b-5p[19]大鼠LPSmiR-495[20]小鼠LPSmiR-802[21]小鼠LPSmiR-92a[22]小鼠LPSmiR-34b-5p[23]小鼠LPSmiR-199a[24]大鼠光气miR-28-5p[25]大鼠重物致伤miR-124-3p[26]大鼠LPSmiRNA-126[27]小鼠LPSmiRNA-126-3p[28]小鼠LPSmiR-10a/b-5p[29]
LPS: 脂多糖;TAC: 牛黄胆酸钠;CLP: 盲肠结扎穿孔术
2 与肺泡上皮细胞相关miRNAs
肺泡上皮细胞是肺泡血管屏障的主要组成部分,损伤会导致屏障功能下降,并且由于肺泡上皮完整性的破坏以及表面活性物质合成减少导致肺水的清除障碍,最终导致肺纤维化。miRNAs通过介导多种信号通路调控细胞增殖、凋亡及炎症因子表达,从而影响肺泡上皮细胞功能。
表2 miRNAs在各种细胞内参与调控ALI
Tab.2 miRNAs are involved in the regulation of ALI in various cells
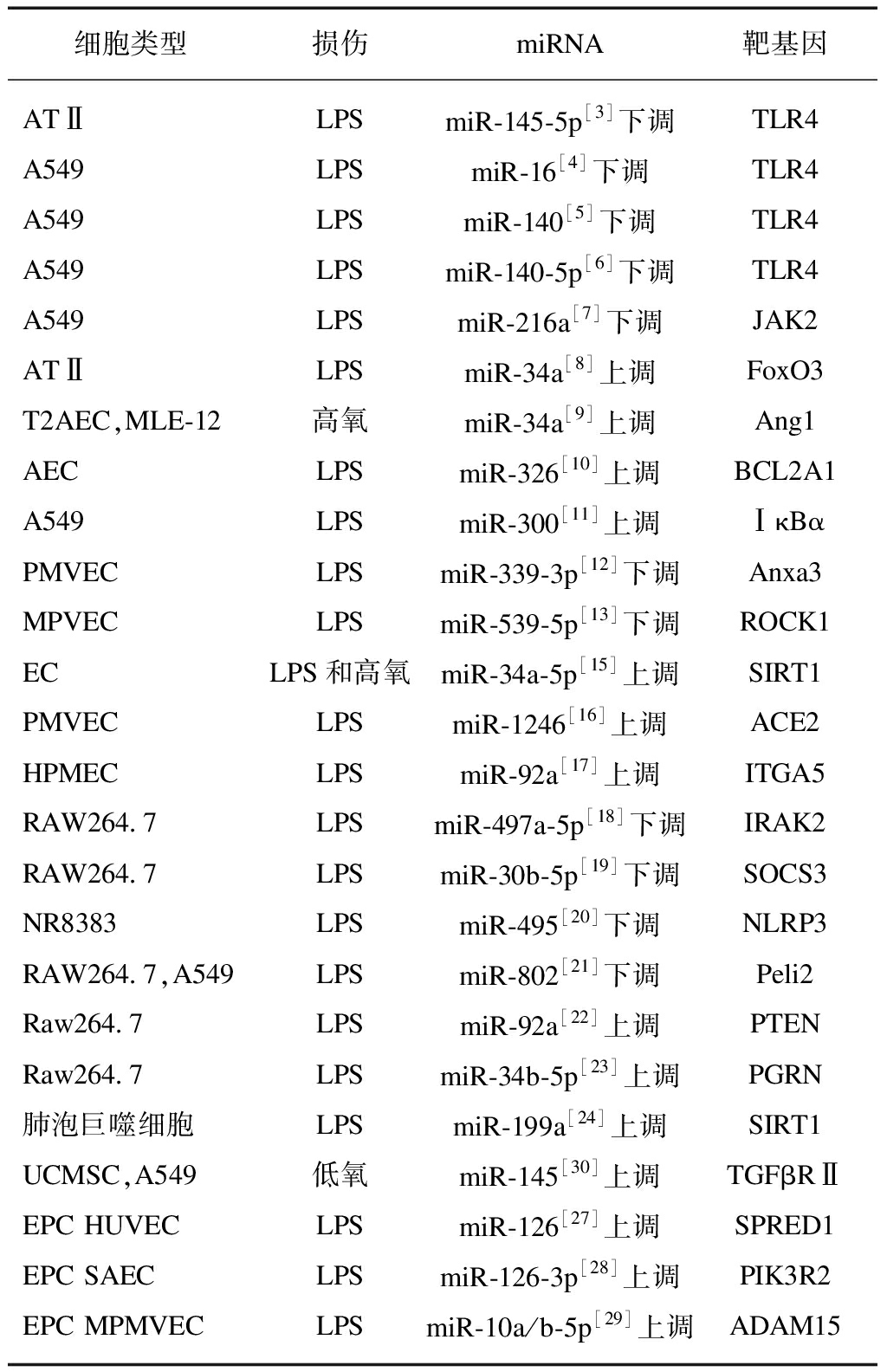
细胞类型损伤miRNA靶基因ATⅡLPSmiR-145-5p[3]下调TLR4A549LPSmiR-16[4]下调TLR4A549LPSmiR-140[5]下调TLR4A549LPSmiR-140-5p[6]下调TLR4A549LPSmiR-216a[7]下调JAK2ATⅡLPSmiR-34a[8]上调FoxO3T2AEC,MLE-12高氧miR-34a[9]上调Ang1AECLPSmiR-326[10]上调BCL2A1A549LPSmiR-300[11]上调ⅠκBαPMVECLPSmiR-339-3p[12]下调Anxa3MPVECLPSmiR-539-5p[13]下调ROCK1ECLPS和高氧miR-34a-5p[15]上调SIRT1PMVECLPSmiR-1246[16]上调ACE2HPMECLPSmiR-92a[17]上调ITGA5RAW264.7LPSmiR-497a-5p[18]下调IRAK2RAW264.7LPSmiR-30b-5p[19]下调SOCS3NR8383LPSmiR-495[20]下调NLRP3RAW264.7,A549LPSmiR-802[21]下调Peli2Raw264.7LPSmiR-92a[22]上调PTENRaw264.7LPSmiR-34b-5p[23]上调PGRN肺泡巨噬细胞LPSmiR-199a[24]上调SIRT1UCMSC,A549低氧miR-145[30]上调TGFβRⅡEPC HUVECLPSmiR-126[27]上调SPRED1EPC SAECLPSmiR-126-3p[28]上调PIK3R2EPC MPMVECLPSmiR-10a/b-5p[29]上调ADAM15
LPS: 脂多糖
2.1 保护性miRNAs
在脂多糖(lipopolysaccharide, LPS)诱导的肺泡Ⅱ型上皮细胞(alveolar type Ⅱ epithelial cell, ATⅡ)中miR-145-5P显著下调[3],人癌系肺泡Ⅱ型上皮细胞(adenocarcinomic human alveolar epithelial cell, A549)中miR-16[4]、miR-140[5]和miR-140-5p[6]也明显下降,并且上述miRNAs均与Toll样受体4(toll like receptor 4, TLR4)mRNA的3’-UTR结合。TLR4定位于细胞膜和细胞质,是LPS的模式识别受体[31],与LPS结合后触发信号级联效应,激活了下游NF-κB通路[32],因此过表达miR-145-5p、miR-16、miR-140及miR-140-5p可以抑制TLR4的表达,阻断NF-κB通路的活化,降低炎症因子的表达,从而减轻ALI。
也有研究发现在高氧诱导的肺泡 Ⅱ 型上皮细胞(type 2 alveolar epithelial cell, AECⅡ、T2AEC)中miR-16表达明显下降[33],且LPS诱导的A549细胞中miR-216a[7]的表达也显著下调。过表达miR-16促进细胞增殖并抑制其凋亡,这可能与TGF-β/Smad2和JAK/STAT3通路有关[33]。而miR-216a是通过靶向JAK激酶2(janus kinase, JAK2)来调节JAK2/STAT3和NF-κB信号转导来抑制细胞凋亡、自噬及炎症因子释放[7]。Kloss等[34]发现TGF-β/Smad通路主要调节细胞生长、增殖、分化、凋亡和迁移等过程。而JAK/STAT通路调控细胞因子、黏附分子和炎症因子转录[35]。所以miRNAs可以通过介导多种信号通路(TGF-β/Smad、JAK/STAT和NF-κB)来缓解ALI。
2.2 损伤性miRNAs
在LPS诱导ATⅡ细胞[8]和暴露于高氧的T2AEC、小鼠肺上皮细胞-12(murine lung epithelial-12, MLE12)中[19]发现miR-34a表达明显升高,结果证实miR-34a可以与自噬相关基因叉头框转录因子O3(forkhead box O3, FoxO3)[8]及血管生成素1(angiopoietin-1,Ang1)[9]的3’-UTR结合。既往研究表明FoxO3能够抑制NF-κB的活性[36],而Fang等[37]发现Ang1可以抑制人T2AEC中的NF-κB活性来恢复上皮细胞对蛋白质的通透性。所以,miR-34a通过靶控FoxO3或Ang1介导NF-κB通路来调控肺泡上皮细胞功能。
LPS诱导的AEC模型中,靶向B淋巴细胞瘤2相关蛋白A1(B cell lymphoma 2 related A1, BCL2A1)基因的miR-326激活了NF-κB信号轴[10],另一项研究发现LPS处理的A549细胞中miR-300靶控抑制性κBα蛋白(inhibitor kappa B α, ⅠκBα),活化了NF-κB通路[11]。而在静息状态下,NF-κB二聚体与IκB蛋白结合后存留于胞质中[38],当肺损伤后,细胞受到外源性刺激,NF-κB活化进入细胞核启动了炎症反应[39]。结果证明过表达miR-326和miR-300都可通过介导NF-κB信号通路导致促炎因子表达增加,并促进肺泡上皮细胞凋亡,而抑制miR-326和miR-300的表达则可以缓解急性肺损伤。
3 与血管内皮细胞相关miRNAs
ALI导致血管内皮细胞骨架蛋白解聚,细胞间连接松散,最终导致血管壁通透性增加及单核细胞、淋巴细胞、多核细胞等炎性细胞聚集。miRNAs靶向血管内皮细胞的mRNAs,参与内皮细胞内稳态的改变,包括对细胞周期、凋亡、细胞层通透性和炎症信号的调节,从而调控肺损伤相关内皮细胞的功能。
3.1 保护性miRNAs
LPS诱导肺微血管内皮细胞后发现miR-339-3p、miR-539-5p和miR-33均低表达。研究证实miR-339-3p靶向膜联蛋白A3(annexin A3, Anxa3),抑制AKT/mTOR通路[12],而Anxa3参与调控细胞骨架蛋白的相互作用、细胞分化、增殖、凋亡和炎症反应等[40],且AKT/mTOR通路也调控细胞凋亡和炎症反应[41]。荧光素酶报告基因证实Rho相关卷曲螺旋形成蛋白激酶1(Rho associated coiled coil conta-ining protein kinase 1, ROCK1)是miR-539-5p的靶基因[13],而ROCK1与ALI的氧化应激和细胞凋亡有关[42]。因此miR-339-3p和miR-539-5p都减少急性肺损伤中的细胞凋亡和炎症因子的表达水平。miR-33与受体相互作用蛋白140表达呈负相关[14],受体相互作用蛋白140是NF-κB辅助激活物,通过招募环磷酸腺苷反应元件结合蛋白(cAMP response element binding protein, CREB)调节促炎因子的产生[43],过表达miR-33减轻了ALI的炎症反应。
3.2 损伤性miRNAs
LPS和高氧刺激内皮细胞(endothelial cell, EC)后,发现miR-34a-5p介导的内皮功能障碍与组蛋白去乙酰化酶1(sirtuin type 1, SIRT1)表达降低和p53的表达升高有关[15],SIRT1通过介导p53促使凋亡蛋白Bax进入线粒体,导致线粒体氧化应激损伤[44-45]。LPS显著刺激了肺微血管内皮细胞中miR-1246和miR-92a的表达。由于miR-1246靶向血管紧张素转化酶2(angiotensin-converting enzyme 2, ACE2)基因[16],ACE2又是通过裂解AngⅡ 产生血管紧张素1-7来使AngⅡ 失活[46],而AngⅡ 与受体结合调节氧化应激、炎症反应和细胞凋亡来缓解急性肺损伤[47]。结果证明抑制miR-34a-5p或miR-1246的表达可抑制氧化应激,减轻内皮细胞凋亡,降低炎症因子表达水平。抑制miR-92a,可以增加其靶基因整联蛋白α5(integrin α 5, ITGA5)的表达[17],ITGA5在细胞黏附、增殖、迁移中起着关键作用[48]。结果也证实ITGA5明显增加肺微血管内皮细胞的迁移,增强血管生成,改善内皮细胞功能,并减少促炎因子释放。研究显示miR-92a与ITGA5的上述作用可能与PI3K/AKT、NF-κB通路相关[49]。
4 与巨噬细胞相关miRNAs
巨噬细胞具有吞噬、分泌等多种功能,在急性肺损伤早期,分泌多种细胞因子启动级联炎性反应。大量的研究表明miRNAs参与免疫调节,单核细胞的发育、分化、增殖及等中心环节。
4.1 保护性miRNAs
在LPS诱导小鼠腹腔巨噬细胞(mouse peritoneal macrophage cell, RAW264.7)中,miR-497和miR-30b-5p的表达下降。抑制miR-497的表达增加了白介素1受体相关激酶2(interleukin-1 receptor-associated kinase 2, IRAK2)的表达,却抑制了NF-κB通路蛋白的表达[18]。在TLR早期,IRAK1和IRAK2都发挥重要作用,但在后期IRAK2则起着关键作用[50]。既往研究发现IRAK2参与了IL-1诱导的NF-κB通路的激活[51]。miR-30b-5p可以和细胞因子信号转导抑制因子3(suppressor of cytokine signaling 3, SOCS3)的3’-UTR结合[19],而SOCS家族介导多种细胞因子的产生[52],且SOCS3负调控JAK/STAT3通路介导肺巨噬细胞炎症[53]。综上所述,miR-497和miR-30b-5p都能抑制ALI炎症因子的表达。
LPS导致肺泡巨噬细胞中miR-495和miR-802的表达显著下调。研究发现miR-495基因启动子甲基化导致miR-495降解,活化炎症小体3(nod like receptor family pyrin domain containing 3, NLRP3),而抑制miR-495的降解可以减轻肺泡巨噬细胞的炎症反应和焦亡,缓解ALI[20]。You等[21]证实miR-802通过靶控皮层E3泛素蛋白连接酶家族2(pellino E3 ubiquitin protein ligase family member 2, Peli2)来改善肺损伤。Peli2通过促进LPS诱导NLRP3的泛素化来介导NLRP3的激活[54]。而NLRP3与肺部炎性疾病发生发展密切相关[55]。总之,miR-495和miR-802都可以通过NLRP3信号轴介导炎症反应,改善ALI。
4.2 损伤性miRNAs
在LPS诱导RAW264.7中发现miR-92a及miR-34b-5p的表达明显上调。miR-92a通过与10号染色体上的磷酸酯酶与张力蛋白同源物(gene of phosphate and tension homology deleted on chromosome ten, PTEN)的3’-UTR结合并阻断PTEN/AKT/NF-κB信号传导途径来抑制炎症反应[22]。PTEN在增殖、凋亡和炎症等多种过程中起关键作用,并能够抑制PI3K/AKT信号通路[56]。研究证实原颗粒蛋白(progranulin, PGRN)是miR-34b-5p的功能靶标[23],并在炎症和细胞凋亡等病理过程中发挥关键性作用[57-58]。肺泡巨噬细胞中miR-199a直接靶控SIRT1基因[24],SIRT1在各种类型的细胞中具有抗炎、抗氧化、抑制DNA损伤和减少细胞凋亡的作用[59-60]。因此,通过抑制miR-92a、miR-34b-5p和miR-199a的表达,减轻ALI中过度的炎症反应和细胞凋亡,提高ALI小鼠生存率。
5 与间充质干细胞相关miRNAs
间充质干细胞(mesenchymal stem cell, MSC)是一种具有多向分化潜能的成体干细胞,可分化为成骨细胞、软骨细胞、脂肪细胞和肺泡细胞等多种体细胞系。MSC调节免疫反应并释放旁分泌因子,以维持屏障功能,促进组织修复并再生新组织,从而缓解ALI,而这种作用主要是由MSC衍生的外泌体包含的miRNAs引起的,而miRNAs在不同组织、不同细胞类型和不同发育阶段表达量不同,在干细胞自我更新、增殖、分化、组织发育等细胞过程中发挥不同的调控作用。
脐带间充质干细胞(umbilical cord mesenchymal stem cell, UCMSC)与A549在低氧条件下共同培养,发现缺氧能够诱导UCMSC分化为ATⅡ细胞,这对于ALI的上皮再形成和恢复至关重要,主要是由miR-145通过靶向TGF-β受体Ⅱ(transforming growth factor-β receptorⅡ, TGFβRⅡ)调控的[30],TGFβRⅡ结合后激活了TGF-β信号。光气暴露导致ALI后,用超速离心法得到的外泌体中包含的miR-28-5p通过PI3K/AKT信号通路促进MSC的增殖及迁移并增加了IL-10的分泌,增强了旁分泌因子如: 血管内皮生长因子(vascular endothelial growth factor, VEGF)、肝细胞生长因子(hepatocyte growth factor, HGF)、抗菌肽(cathelicidin LL-37, LL-37)和Ang 1的表达[25]。使用重物致伤法建立大鼠创伤性ALI模型,然后注入MSC衍生的外泌体,在外泌体中大量表达miR-124-3p直接靶向嘌呤能受体P2X配体门控离子通道7(purinergic receptor P2X ligand-gated ion channel 7, P2X7)[26],而P2X7作为嘌呤能受体,与人类炎症和应激反应密切相关[61]。P2X7敲减抑制炎症反应而对ALI起到保护作用[62]。
6 与内皮祖细胞相关miRNAs
内皮祖细胞(endothelial progenitor cell, EPC)作为成熟内皮细胞的前体细胞,在维持内皮完整、促进血管新生、保护器官功能中发挥重要作用。EPC减轻脓毒血症肺损伤是通过内皮祖细胞衍生的外泌体(endothelial progenitor cell-exosomes, EPC-Exos)包含miRNAs决定的。
LPS诱导大鼠模型后,用EPC-Exos移植到大鼠体内,结果发现miR-126在EPC-Exos中富集,而miR-126又通过抑制其靶点快速发育生长因子同源蛋白1(sprouty-related enabled/vasodilator-stimulated pho-sphoprotein homology 1 domain 1, SPRED1)的表达,激活了RAF/ERK信号通路,增强了人脐静脉内皮细胞(human umbilical vein endothelial cell, HUVEC)的增殖、迁移和管状结构形成[27]。研究发现小气道上皮细胞(small airway epithelial cell, SAEC)中miRNA-126-3p可靶向磷酸肌醇-3-激酶调节亚基2(phosphoinositide-3-kinase regulatory subunit 2, PIK3R2),从而抑制高速泳动族蛋白B1(high mo-bility group box-1, HMGB1)和通透性因子VEGFα的表达[28]。在LPS诱导的ALI小鼠肺微血管内皮细胞(mouse pulmonary microvascular endothelial cell, MPMVEC)和EPC中,增加miR-10a/b-5p的表达或降低靶蛋白整合素金属蛋白酶(a disintegrin and metalloproteinase15, ADAM15)的表达,促进MPMVEC增殖而发挥治疗ALI的作用[29]。敲除ADAM15可以降低肺高通透性和缓解ALI,表明ADAM15在ALI中是一种促炎蛋白[63]。抑制了ADAM15的表达改善了ALI的炎症反应。
7 与COVID-19相关miRNAs
自2019年12月以来,新型冠状病毒(severe acute respiratory syndrome coronavirus 2, SARS-CoV-2)引起的新型冠状病毒肺炎(coronavirus disease 2019, COVID-19)正在全球迅速蔓延。新型冠状病毒传染性强,死亡率高,同时症状隐匿,极大威胁人类生命健康。SARS-CoV-2在人类中的致病性是由于重要生理途径改变,包括与“细胞因子风暴”和广泛的肺部病理学改变相关。免疫应答对于控制SARS-CoV-2感染至关重要,但过度反应反而导致细胞损伤。miRNAs在天然免疫和获得性免疫中调节免疫细胞的分化、发育和激活起着关键作用。
多项研究分析了SARS-CoV-2基因组,以确定哪些区域可以作为病毒编码的miRNA种子海绵,潜在地与人miRNA种子位点结合,并阻止其与天然靶标相互作用,从而缓解天然miRNA抑制的区域。Guterres等[64]发现34个miRNA为顺义病毒RNA,45个miRNA为反义病毒RNA,它们可以与某些关键的SARS-CoV-2基因紧密结合。miRNA的破坏和功能异常可能会扰乱免疫反应并刺激炎性细胞因子的释放,从而改变细胞对病毒感染的反应。
8 展 望
目前ALI的有效防治策略仍然是当前重点和难点问题,而miRNA可通过影响其靶基因,调控肺泡上皮细胞、毛细血管内皮细胞及巨噬细胞功能,在ALI或ARDS发挥重要作用。此外,miRNA可作为生物标记志物,在ALI或ARDS不同病程和病情的诊断中作为重要参考指标。由于机体调控网络的复杂性,同种或不同种细胞中,不同的miRNA可能调控同一个信号通路,同时在一种或多种细胞中同一个miRNA可能参与不同信号通路的调控,由于ALI或ARDS的病情进展迅速、在不同时间点呈现不同病理生理特点,因此miRNA可能在不同时间点或不同细胞中起着完全相反的作用。miRNA与靶基因结合影响多种信号通路,调控细胞凋亡、血管生成和免疫等多种病理生理过程,此外miRNA在不同细胞、组织及各种体液中都稳定存在,故而特异性升高或降低利于病情发展的miRNA的表达可能成为ALI新的治疗方法。miRNA现处于实验室研究阶段,暂时还没有miRNA用于临床试验,有待进一步探索,为临床应用提供坚实基础。
[1] BELLANI G, LAFFEY J G, PHAM T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries[J]. JAMA, 2016,315(8): 788-800.
[2] CARTHEW R W, SONTHEIMER E J. Origins and Mechanisms of miRNAs and siRNAs[J]. Cell, 2009,136(4): 642-655.
[3] YU Y L, YU G, DING Z Y, et al. Overexpression of miR-145-5p alleviated LPS-induced acute lung injury[J]. J Biol Regul Homeost Agents, 2019,33(4): 1063-1072.
[4] YANG Y, YANG F, YU X Q, et al. miR-16 inhibits NLRP3 inflammasome activation by directly targeting TLR4 in acute lung injury[J]. Biomed Pharmacother, 2019,112: 108664.
[5] LI X Y, WANG J, WU H S, et al. Reduced peripheral blood miR-140 may be a biomarker for acute lung injury by targeting Toll-like receptor 4(TLR4)[J]. Exp Ther Med, 2018,16(4): 3632-3638.
[6] YANG Y, LIU D D, XI Y, et al. Upregulation of miRNA-140-5p inhibits inflammatory cytokines in acute lung injury through the MyD88/NF-κB signaling pathway by targeting TLR4[J]. Exp Ther Med, 2018,16(5): 3913-3920.
[7] KONG F P, SUN Y Q, SONG W K, et al. MiR-216a alleviates LPS-induced acute lung injury via regulating JAK2/STAT3 and NF-κB signaling[J]. Hum Cell, 2020,33(1): 67-78.
[8] SONG L, ZHOU F L, CHENG L J, et al. MicroRNA-34a suppresses autophagy in alveolar type Ⅱ epithelial cells in acute lung injury by inhibiting FoxO3 expression[J]. Inflammation, 2017,40(3): 927-936.
[9] SYED M, DAS P, PAWAR A, et al. Hyperoxia causes miR-34a-mediated injury via angiopoietin-1 in neonatal lungs[J]. Nat Commun, 2017,8(1): 1173.
[10] WU C T, HUANG Y, PEI Z Y, et al. MicroRNA-326 aggravates acute lung injury in septic shock by mediating the NF-κB signaling pathway[J]. Int J Biochem Cell Biol, 2018,101: 1-11.
[11] CAO W, DAI H, YANG S, et al. Increased serum miR-300 level serves as a potential biomarker of lipopolysaccharide-induced lung injury by targeting ⅠκBα[J]. Pharmazie, 2017,72(1): 5-9.
[12] WU X M, JI K Q, WANG H Y, et al. MicroRNA-339-3p alleviates inflammation and edema and suppresses pulmonary microvascular endothelial cell apoptosis in mice with severe acute pancreatitis-associated acute lung injury by regulating Anxa3 via the Akt/mTOR signaling pathway[J]. J Cell Biochem, 2018,119(8): 6704-6714.
[13] MENG L, CAO H H, WAN C H, et al. MiR-539-5p alleviates Sepsis-induced acute lung injury by targeting ROCK1[J]. Folia Histochem Cytobiol, 2019,57(4): 168-178.
[14] LI H, HOU H, LIU S, et al. miR-33 and RIP140 participate in LPS-induced acute lung injury[J]. Turk J Med Sci, 2019,49(1): 422-428.
[15] SHAH D, DAS P, ALAM M A, et al. MicroRNA-34a promotes endothelial dysfunction and mitochondrial-mediated apoptosis in murine models of acute lung injury[J]. Am J Respir Cell Mol Biol, 2019,60(4): 465-477.
[16] FANG Y, GAO F Y, HAO J, et al. microRNA-1246 mediates lipopolysaccharide-induced pulmonary endothelial cell apoptosis and acute lung injury by targeting angiotensin-converting enzyme 2[J]. Am J Transl Res, 2017,9(3): 1287-1296.
[17] XU F, ZHOU F. Inhibition of microRNA-92a ameliorates lipopolysaccharide-induced endothelial barrier dysfunction by targeting ITGA5 through the PI3K/Akt signaling pathway in human pulmonary microvascular endothelial cells[J]. Int Immunopharmacol, 2020,78: 106060.
[18] GUO S, CHEN Y, LIU J F, et al. miR-497a-5p attenuates lipopolysaccharide-induced inflammatory injury by targeting IRAK2[J]. J Cell Physiol, 2019,234(12): 22874-22883.
[19] ZHOU T, CHEN Y L. The functional mechanisms of miR-30b-5p in acute lung injury in children[J]. Med Sci Monit, 2019,25: 40-51.
[20] YING Y G, MAO Y, YAO M. NLRP3 inflammasome activation by MicroRNA-495 promoter methylation may contribute to the progression of acute lung injury[J]. Mol Ther Nucleic Acids, 2019,18: 801-814.
[21] YOU Q H, WANG J M, JIA D, et al. MiR-802 alleviates lipopolysaccharide-induced acute lung injury by targeting Peli2[J]. Inflamm Res, 2020,69(1): 75-85.
[22] FU L M, ZHU P, QI S L, et al. MicroRNA-92a antagonism attenuates lipopolysaccharide(LPS)-induced pulmonary inflammation and injury in mice through suppressing the PTEN/AKT/NF-κB signaling pathway[J]. Biomed Pharmacother, 2018,107: 703-711.
[23] XIE W, LU Q C, WANG K L, et al. miR-34b-5p inhibition attenuates lung inflammation and apoptosis in an LPS-induced acute lung injury mouse model by targeting progranulin[J]. J Cell Physiol, 2018,233(9): 6615-6631.
[24] LIU Y, GUAN H, ZHANG J L, et al. Acute downregulation of miR-199a attenuates Sepsis-induced acute lung injury by targeting SIRT1[J]. Am J Physiol Cell Physiol, 2018,314(4): C449-C455.
[25] XU N, HE D, SHAO Y, et al. Lung-derived exosomes in phosgene-induced acute lung injury regulate the functions of mesenchymal stem cells partially via miR-28-5p[J]. Biomed Pharmacother, 2020,121: 109603.
[26] LI Q C, LIANG Y, SU Z B. Prophylactic treatment with MSC-derived exosomes attenuates traumatic acute lung injury in rats[J]. Am J Physiol Lung Cell Mol Physiol, 2019,316(6): L1107-L1117.
[27] WU X, LIU Z L, HU L J, et al. Exosomes derived from endothelial progenitor cells ameliorate acute lung injury by transferring miR-126[J]. Exp Cell Res, 2018,370(1): 13-23.
[28] ZHOU Y, LI P F, GOODWIN A J, et al. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury[J]. Crit Care, 2019,23(1): 44.
[29] JIN Y, YANG C, SUI X T, et al. Endothelial progenitor cell transplantation attenuates lipopolysaccharide-induced acute lung injury via regulating miR-10a/b-5p[J]. Lipids Health Dis, 2019,18(1): 136.
[30] LI Y, SHI X, YANG L M, et al. Hypoxia promotes the skewed differentiation of umbilical cord mesenchymal stem cells toward type Ⅱ alveolar epithelial cells by regulating microRNA-145[J]. Gene, 2017,630: 68-75.
[31] SHUTO T, KATO K, MORI Y, et al. Membrane-anchored CD14 is required for LPS-induced TLR4 endocytosis in TLR4/MD-2/CD14 overexpressing CHO cells[J]. Biochem Biophys Res Commun, 2005,338(3): 1402-1409.
[32] ZHANG J J, DOU W, ZHANG E Y, et al. Paeoniflorin abrogates DSS-induced colitis via a TLR4-dependent pathway[J]. Am J Physiol Gastrointest Liver Physiol, 2014,306(1): G27-G36.
[33] LI Z X, JIANG W J, WU G, et al. miR-16 inhibits hyperoxia-induced cell apoptosis in human alveolar epithelial cells[J]. Mol Med Rep, 2018,17(4): 5950-5957.
[34] KLOSS C C, LEE J, ZHANG A, et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication[J]. Mol Ther, 2018,26(7): 1855-1866.
[35] CHEN W D, ZHANG J L, WANG X Y, et al. The JAK2/STAT3 signaling pathway is required for inflammation and cell death induced by cerulein in AR42J cells[J]. Eur Rev Med Pharmacol Sci, 2019,23(4): 1770-1777.
[36] LIN L, HRON J D, PENG S L. Regulation of NF-κB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a[J]. Immunity, 2004,21(2): 203-213.
[37] FANG X H, NEYRINCK A P, MATTHAY M A, et al. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type Ⅱ cells by secretion of angiopoietin-1[J]. J Biol Chem, 2010,285(34): 26211-26222.
[38] HOFFMANN A, NATOLI G, GHOSH G. Transcriptional regulation via the NF-κB signaling module[J]. Oncogene, 2006,25(51): 6706-6716.
[39] ZHANG Q, HUI W, LITHERLAND G J, et al. Differential Toll-like receptor-dependent collagenase expression in chondrocytes[J]. Ann Rheum Dis, 2008,67(11): 1633-1641.
[40] TONG M, FUNG T M, LUK S T, et al. ANXA3/JNK signaling promotes self-renewal and tumor growth, and its blockade provides a therapeutic target for hepatocellular carcinoma[J]. Stem Cell Reports, 2015,5(1): 45-59.
[41] PONNURANGAM S, STANDING D, RANGARAJAN P, et al. Tandutinib inhibits the Akt/mTOR signaling pathway to inhibit colon cancer growth[J]. Mol Cancer Ther, 2013,12(5): 598-609.
[42] CINEL I, ARK M, DELLINGER P, et al. Involvement of Rho kinase(ROCK) in Sepsis-induced acute lung injury[J]. J Thorac Dis, 2012,4(1): 30-39.
[43] ZSCHIEDRICH I, HARDELAND U, KRONES-HERZIG A, et al. Coactivator function of RIP140 for NF-κB/RelA-dependent cytokine gene expression[J]. Blood, 2008,112(2): 264-276.
[44] DAI C Q, LUO T T, LUO S C, et al. p53 and mitochondrial dysfunction: novel insight of neurodegenerative diseases[J]. J Bioenerg Biomembr, 2016,48(4): 337-347.
[45] VASEVA A V, MOLL U M. The mitochondrial p53 pathway[J]. Biochim Biophys Acta, 2009,1787(5): 414-420.
[46] VICKERS C, HALES P, KAUSHIK V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase[J]. J Biol Chem, 2002,277(17): 14838-14843.
[47] SHARMA N, MALEK V, MULAY S R, et al. Angiotensin Ⅱ type 2 receptor and angiotensin-converting enzyme 2 mediate ischemic renal injury in diabetic and non-diabetic rats[J]. Life Sci, 2019,235: 116796.
[48] HYNES R O. Integrins: bidirectional, allosteric signaling machines[J]. Cell, 2002,110(6): 673-687.
[49] HATTORI Y, HATTORI S, KASAI K. Lipopolysaccharide activates Akt in vascular smooth muscle cells resulting in induction of inducible nitric oxide synthase through nuclear factor-κ B activation[J]. Eur J Pharmacol, 2003,481(2-3): 153-158.
[50] KAWAGOE T, SATO S, MATSUSHITA K, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2[J]. Nat Immunol, 2008,9(6): 684-691.
[51] MUZIO M, NI J, FENG P, et al. IRAK(Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling[J]. Science, 1997,278(5343): 1612-1615.
[52] KREBS D L, HILTON D J. SOCS proteins: negative regulators of cytokine signaling[J]. Stem Cells, 2001,19(5): 378-387.
[53] CHAVES DE SOUZA J A, NOGUEIRA A V, CHAVES DE SOUZA P P, et al. SOCS3 expression correlates with severity of inflammation, expression of proinflammatory cytokines, and activation of STAT3 and p38 MAPK in LPS-induced inflammation in vivo[J]. Mediators Inflamm, 2013,2013: 650812.
[54] HUMPHRIES F, BERGIN R, JACKSON R, et al. The E3 ubiquitin ligase pellino 2 mediates priming of the NLRP3 inflammasome[J]. Nat Commun, 2018,9(1): 1560.
[55] COLL R C, ROBERTSON A A, CHAE J J, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases[J]. Nat Med, 2015,21(3): 248-255.
[56] HOPKINS B D, HODAKOSKI C, BARROWS D, et al. PTEN function: the long and the short of it[J]. Trends Biochem Sci, 2014,39(4): 183-190.
[57] MA Y B, MATSUWAKI T, YAMANOUCHI K, et al. Progranulin protects hippocampal neurogenesis via suppression of neuroinflammatory responses under acute immune stress[J]. Mol Neurobiol, 2017,54(5): 3717-3728.
[58] KANSAL S, BHATNAGAR A, AGNIHOTRI N. Fish oil suppresses cell growth and metastatic potential by regulating PTEN and NF-κB signaling in colorectal cancer[J]. PLoS One, 2014,9(1): e84627.
[59] COHEN H Y, MILLER C, BITTERMAN K J, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase[J]. Science, 2004,305(5682): 390-392.
[60] FINKEL T, DENG C X, MOSTOSLAVSKY R. Recent progress in the biology and physiology of sirtuins[J]. Nature, 2009,460(7255): 587-591.
[61] FAROOQ R K, TANTI A, AINOUCHE S, et al. A P2X7 receptor antagonist reverses behavioural alterations, microglial activation and neuroendocrine dysregulation in an unpredictable chronic mild stress(UCMS) model of depression in mice[J]. Psychoneuroendocrinology, 2018,97: 120-130.
[62] GALAM L, RAJAN A, FAILLA A, et al. Deletion of P2X7 attenuates hyperoxia-induced acute lung injury via inflammasome suppression[J]. Am J Physiol Lung Cell Mol Physiol, 2016,310(6): L572-L581.
[63] SUN C X, BEARD R S Jr, MCLEAN D L, et al. ADAM15 deficiency attenuates pulmonary hyperpermeability and acute lung injury in lipopolysaccharide-treated mice[J]. Am J Physiol Lung Cell Mol Physiol, 2013,304(3): L135-L142.
[64] GUTERRES A, DE AZEREDO LIMA C H, MIRANDA R L, et al. What is the potential function of microRNAs as biomarkers and therapeutic targets in COVID-19?[J]. Infect Genet Evol, 2020,85: 104417.