外泌体是细胞分泌的纳米级囊泡,广泛存在于血液、尿液、乳汁等体液中[1-6]。研究发现牛奶中含有大量的外泌体,且外泌体可以保护其内含的microRNAs[7-11]。超速离心法是目前获取牛奶外泌体最常用的的方法之一[12],但不同的研究获取外泌体的离心力不尽相同[13-16]。本研究拟通过对不同离心力下获取的牛奶外泌体进行表征,探究获取牛奶外泌体的最适离心力。
研究已发现牛奶外泌体具有促进肠上皮细胞增殖等作用[13,17]。但是,其在胃肠道的稳定性仍不十分明确,而且消化后的牛奶外泌体是否仍对细胞增殖具有促进作用尚无人探究。故本课题将在之前研究[16]的基础上,对牛奶外泌体在胃肠道的稳定性进行进一步的验证,并初步探究牛奶消化前后外泌体对大鼠肠隐窝上皮细胞(IEC-6)增殖的作用。
1 材料与方法
1.1 体外胃肠道消化模型
牛奶的体外消化参照以往研究[16,18-19]中使用的体外胃肠道消化模型进行,实验所用牛奶为从超市购买的某品牌0脂鲜牛奶。参考Tunick等[19]的研究配制模拟胃液(simulated gastric fluid, SGF)和模拟肠液(simulated intestinal fluid, SIF)。具体实验过程如下。
在模拟胃反应阶段,100mL牛奶与50mL SGF混匀,调整pH至1.5,37℃预热30min,置于恒温摇床内,37℃,95r/min反应30min。模拟胃反应阶段结束后,在上述溶液中加入150mL SIF,调整pH至7,37℃预热30min,置于恒温摇床内,37℃,95r/min,反应60min。反应完结束后,60℃水浴10min灭活消化酶以终止反应[16]。然后,将其迅速置于冰上,直至用超速离心法提取外泌体。
因本研究后续将通过细胞增殖实验探究消化前后的牛奶外泌体对细胞增殖的影响,为了排除模拟消化液SGF和SIF中的成分对细胞增殖的影响,本研究除了设置未消化的牛奶组(N组)和体外模型消化组(NX组)外,还设置了空白对照组(ND组),即用纯水代替牛奶在同样条件下进行体外消化。
1.2 获取外泌体的离心方案
将N、NX和ND组样品用贝克曼冷冻离心机7000×g相对离心力(relative centrifugal force, RCF)、 20000×g RCF、50000×g RCF,4℃下各离心60min,以去除脂蛋白、酪蛋白和细胞碎片等,收集上清液,弃沉淀;将收集的上清液转移至新的离心管,70000×g RCF,4℃离心60min,去除剩余的蛋白和大颗粒物质,弃沉淀,收集上清液,用于下一步离心。
不同离心力外泌体的获取步骤为: 100000×g RCF,4℃,离心60min,将上清液转移至新的离心管,130000×g RCF,4℃离心60min,再将上清液160000×g RCF,4℃离心60min。分别用适量PBS溶液溶解收集不同离心力下的沉淀。N组、NX组、ND组样品在100000×g RCF,130000×g RCF,160000×g RCF下获得的外泌体溶液分别标记为N1、N2、N3、NX1、NX2、NX3、ND1、ND2、ND3。
1.3 外泌体表征
外泌体溶液的蛋白测定用上海碧云天生物技术有限公司增强型BCA蛋白浓度测定试剂盒;用Western印迹法分析外泌体标记蛋白CD63和CD9的表达[20-21];动态光散射法(dynamic light scattering, DLS)测定外泌体粒径,选取分散系数(polydispersity index, PDI)和数量粒度分布均值(particle size distribution number mean, PSD N M)两个指标分析外泌体粒径数据[22]。外泌体的形态采用日本日立公司的S-4800扫描电子显微镜观察。
1.4 细胞增殖实验
选取对数生长期的IEC-6细胞,分成4组,即对照组(C组)、N组、NX组和ND组。其中对照组不加任何干预,N、NX分别在培养基中加入50μg/mL不同离心力下获取的消化前、消化后的牛奶外泌体进行培养,ND组则加入与NX组相同体积的离心产物作为对照。在24、48、72h时进行CCK8测定,计算细胞增殖率,实验独立重复3次。此外,为排除血清中外泌体对实验结果的干扰,细胞增殖实验所用血清均为去除外泌体的血清[23]。
1.5 统计学处理
用GraphPad Prism 6.0进行数据分析和作图,数据间的两两比较用t检验进行,同时进行方差齐性检验,数据以![]() 表示。P<0.05为差异有统计学意义。
表示。P<0.05为差异有统计学意义。
2 结 果
2.1 不同离心力下获得的牛奶消化前后外泌体的表征
体外消化后,牛奶外泌体蛋白浓度明显降低,见图1。外泌体标记蛋白CD63和CD9在消化前后的牛奶外泌体中均为阳性,见图2;不含牛奶的空白对照组标记蛋白阴性。
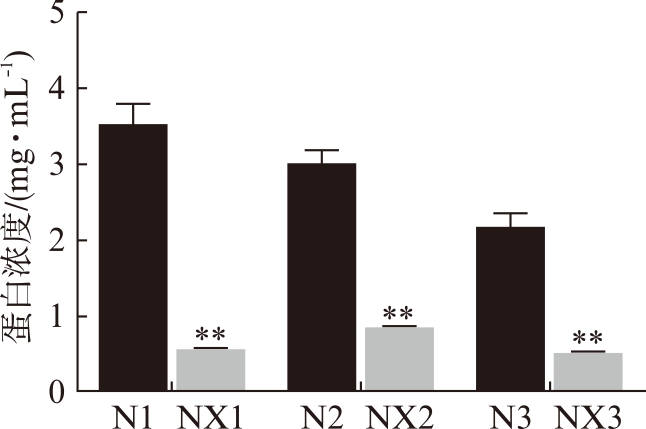
图1 牛奶消化前后外泌体蛋白浓度的变化
Fig.1 The change of bovine exosomes protein
concentration
与N1组相比,**P<0.01
图2 牛奶外泌体标记蛋白表达
Fig.2 Protein markers of bovine milk exosomes
DLS法测得的粒径数据显示,牛奶消化后离心获取的外泌体的数量粒度分布均值和分散系数均大于未经消化处理的牛奶组,消化前后的牛奶外泌体粒径随离心力的增加而降低,见表1、图3。
表1 牛奶外泌体的分散系数和数量粒度分布均值
Tab.1 The PSD and PDI of bovine milk exosomes (n=3)
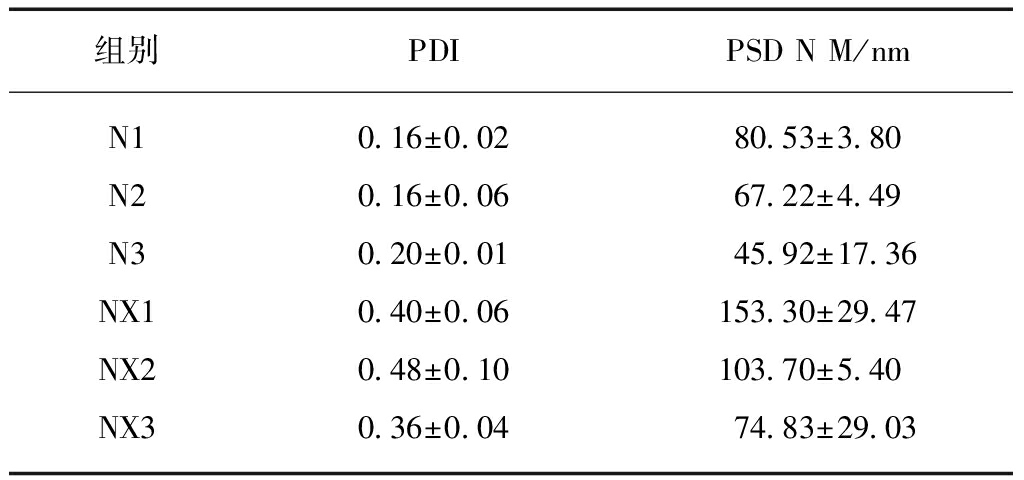
组别PDIPSD N M/nmN10.16±0.0280.53±3.80N20.16±0.0667.22±4.49N30.20±0.0145.92±17.36NX10.40±0.06153.30±29.47NX20.48±0.10103.70±5.40NX30.36±0.0474.83±29.03
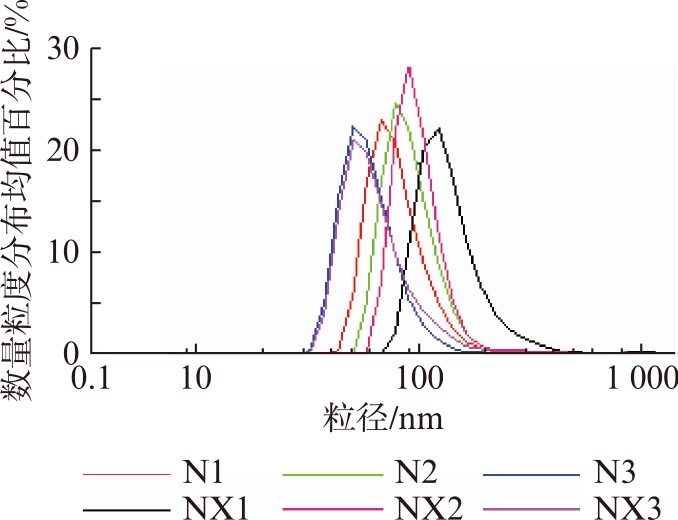
图3 牛奶外泌体的数量粒度分布图
Fig.3 Bovine milk exosomes size distribution
扫描电镜下观察,N1、N2、NX1、NX2组均能观察到类似外泌体的形态结构,但是N3、NX3组粒径过小,形态不规则;电镜下观察N1内含粒径小、形状不规则的杂质,疑似混有杂蛋白,而NX1和NX2则不存在这种现象,见图4。
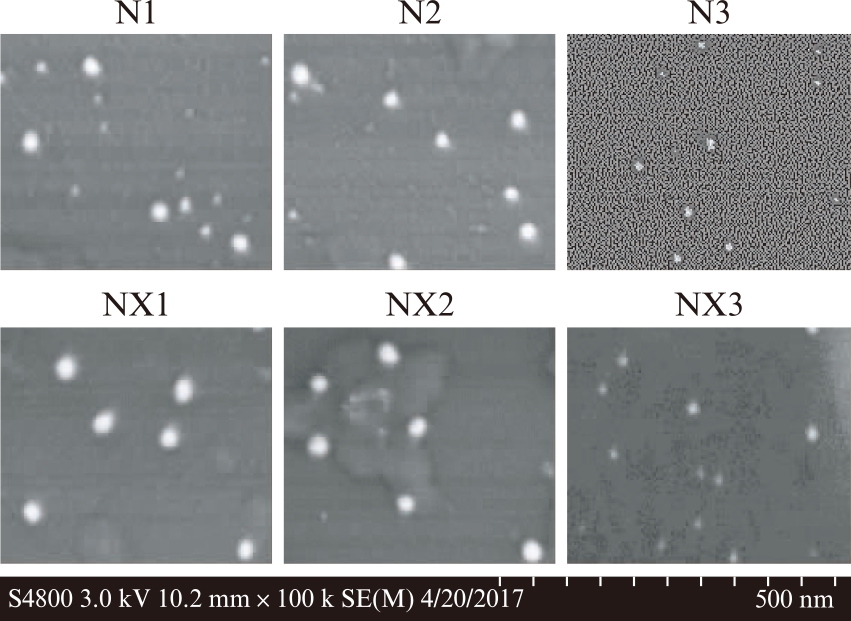
图4 牛奶外泌体的形态
Fig.4 Characterization of bovine milk exosomes
2.2 不同离心力下获得的牛奶消化前后外泌体对IEC-6细胞增殖的影响
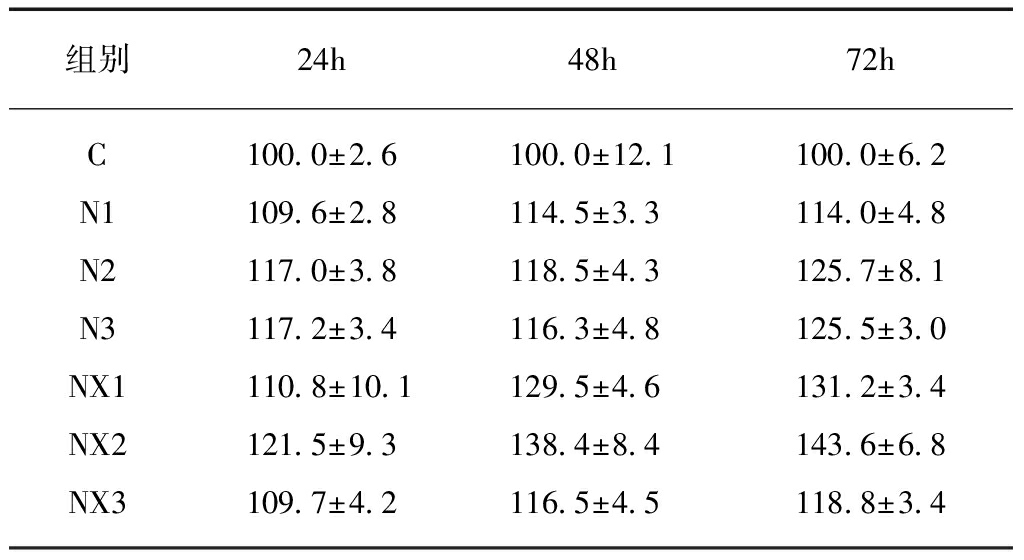
实验结果显示,不同离心力下获得的牛奶消化前后的外泌体处理IEC-6细胞24、48、72h后,细胞增殖率均明显高于对照组,差异有统计学意义(P<0.05)。
在24h时,N2组的细胞增殖率大于N1组(P<0.01);而NX1、NX2和NX3三组间的细胞增殖率差异无统计学意义。同一离心力下获得的N组和NX组的离心产物之间增殖率无明显差别,即N1与NX1、N12与NX2、N3与NX3之间均差异无统计学意义。在48h时,N1、N2、N3三组间细胞增殖率差异无统计学意义。在NX中,NX2组的细胞增殖率明显高于NX1和NX3。同一离心力下获得的N组和NX组离心产物对细胞增殖有明显不同: NX1的增殖率大于N1且NX2的增殖率大于N2(P<0.05)。
在72h时,N2和N3组的细胞增殖率大于N1(P<0.05),NX2组的细胞增殖率大于NX1。NX1的细胞增殖率大于N1且NX2的细胞增殖率大于N2(P<0.05),见表2。
表2 牛奶外泌体对IEC-6细胞增殖的影响
Tab.2 The effect of bovine milk exosomes on
proliferation of IEC-6 cells (n=3)
组别24h48h72hC100.0±2.6100.0±12.1100.0±6.2N1109.6±2.8114.5±3.3114.0±4.8N2117.0±3.8118.5±4.3125.7±8.1N3117.2±3.4116.3±4.8125.5±3.0NX1110.8±10.1129.5±4.6131.2±3.4NX2121.5±9.3138.4±8.4143.6±6.8NX3109.7±4.2116.5±4.5118.8±3.4
3 讨 论
牛奶外泌体的提取主要采用超速离心、试剂盒分离等方法[12],用超速离心法获取外泌体这一途径虽然已被广泛采用,但是,通过超速离心法获取牛奶外泌体的离心方案不尽相同[13,16,22,24]。目前研究多用100000×g至130000×g的最终离心力获得牛奶外泌体。本研究通过对不同离心力获取的外泌体进行表征,探讨获取牛奶外泌体的最适离心力。本研究发现,在N组离心产物中,外泌体标记蛋白CD63和CD9在N1、N2和N3中均呈现阳性,说明不同离心力下获得的离心产物都含有外泌体。但是,结合外泌体的粒径数据和电镜下的形态,可以看出N1的离心产物中含有一定量粒径较小的杂蛋白,这些杂蛋白可能会干扰实验结果。而N2基本上不含粒径较小的杂蛋白;N3的粒径过小且形状不规则,无法断定其内是否混有粒径极小的杂蛋白,且尚未有研究使用160000×g的离心力分离外泌体,故不推荐使用。因此,本研究认为,若不采用采用其他手段(如加入EDTA等物质[24])去除杂蛋白,而单纯采用梯度离心法获取外泌体,100000×g离心后的上清液,再经130000×g离心分离牛奶外泌体是比较合理的离心方案。
牛奶外泌体能耐受胃肠道的消化是其被吸收发挥作用的前提。本研究通过对消化前后的牛奶外泌体进行表征,发现消化后获取的牛奶外泌体粒径大于未消化组,这与之前牛奶外泌体在胃肠道稳定性的研究[16]结果存在差异。但是,结合扫描电镜下观察到的外泌体形态数据,本研究认为造成上述结果的原因是N组离心产物混有较多粒径较小的杂蛋白,从而导致N组的数量粒度分布均值低于NX组。鉴于NX组离心产物在标记蛋白和形态等方面与N组一致,可以推断牛奶中的外泌体经过体外消化后仍有大部分存留。
牛奶外泌体有诸多功能,如促进肠上皮细胞增殖[13,17]及肠道的损伤修复[25],影响哺乳动物免疫和生长发育[11],26],调控人体基因的表达等[27]。消化后的牛奶外泌体是否仍具有未消化的牛奶外泌体的功能目前尚无报道。本课题组在前期研究[22]中发现,牛奶外泌体可通过影响MAPK信号通路的表达促进IEC-6细胞的增殖。本研究发现,消化后的牛奶外泌体仍能促进IEC-6细胞的增殖,NX2组在48、72h的增殖率较高,且NX1和NX2组的增殖率在48、72h均分别明显高于N1和N2组。有研究表明,蛋白酶能够去除外泌体膜上的蛋白[24],进而影响肠细胞对外泌体的摄取。体外消化可能会使牛奶外泌体会发生某些变化,进而导致其对细胞增殖的影响与未消化的牛奶外泌体有明显差异。对于这一猜想还有待于在后续的实验中作进一步的探究和论证。
[1] RECORD M, CARAYON K, POIROT M, et al. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta, 2014,1841(1): 108-120.
[2] SCHNEIDER A, SIMONS M. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res, 2013,352(1): 33-47.
[3] MATHIVANAN S, JI H, SIMPSON R J. Exosomes: extracellular organelles important in intercellular communication. J Proteomics, 2010,73(10): 1907-1920.
[4] SIMONS M, RAPOSO G. Exosomes-vesicular carriers for intercellular communication. Curr Opin Cell Biol, 2009,21(4): 575-581.
[5] RATAJCZAK J, WYSOCZYNSKI M, HAYEK F, et al. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia, 2006,20(9): 1487-1495.
[6] THERY C, ZITVOGEL L, AMIGORENA S. Exosomes: composition, biogenesis and function. Nat Rev Immunol, 2002,2(8): 569-579.
[7] HATA T, MURAKAMI K, NAKATANI H, et al. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun, 2010,396(2): 528-533.
[8] IZUMI H, TSUDA M, SATO Y, et al. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J Dairy Sci, 2015,98(5): 2920-2933.
[9] GU Y, LI M, WANG T, et al. Lactation-related microRNA expression profiles of porcine breast milk exosomes. PLoS One, 2012,7(8): e43691.
[10] ZHOU Q, LI M, WANG X, et al. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci, 2012,8(1): 118-123.
[11] KOSAKA N, IZUMI H, SEKINE K, et al. microRNA as a new immune-regulatory agent in breast milk. Silence, 2010,1(1): 7.
[12] YAMADA T, INOSHIMA Y, MATSUDA T, et al. Comparison of methods for isolating exosomes from bovine milk. J Vet Med Sci, 2012,74(11): 1523-1525.
[13] HOCK A, MIYAKE H, LI B, et al. Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg, 2017,52(5): 755-759.
[14] CHEN T, XI Q Y, YE R S, et al. Exploration of microRNAs in porcine milk exosomes. BMC Genomics, 2014,15: 100.
[15] MUNAGALA R, AQIL F, JEYABALAN J, et al. Bovine milk-derived exosomes for drug delivery. Cancer letters, 2016,371(1): 48-61.
[16] BENMOUSSA A, LEE C H, LAFFONT B, et al. Commercial dairy cow milk microRNAs resist digestion under simulated gastrointestinal tract conditions. Nutrition, 2016,146(11): 2206-2215.
[17] CHEN T, XIE M Y, SUN J J, et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci Rep, 2016,6: 33862.
[18] YANG J, HOTZ T, BROADNAX L, et al. Anomalous uptake and circulatory characteristics of the plant-based small RNA MIR2911. Sci Rep, 2016,6: 26834.
[19] TUNICK M H, REN D X, VAN HEKKEN D L, et al. Effect of heat and homogenization on in vitro digestion of milk. J Dairy Sci, 2016,99(6): 4124-4139.
[20] 赵晨阳,孙维佳,张鹏飞,等.Exosomes的生物学特征及其在细胞间信号转导中的作用.生物物理学报,2013,29(12): 919-924.
[21] 胡馨予,高正良,徐俊.2-DG对神经干细胞增殖和分化影响的研究.同济大学学报(医学版),2016,37(6): 35-40.
[22] YU S, ZHAO Z, SUN L, et al. Fermentation results in quantitative changes in milk-derived exosomes and different effects on cell growth and survival. J Agric Food Chem, 2017,65(6): 1220-1228.
[23] KUSUMA R J, MANCA S, FRIEMEL T, et al. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis. Am J Physiol Cell Physiol, 2016,310(10): C800-807.
[24] WOLF T, BAIER S R, ZEMPLENI J. The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma Caco-2 cells and rat small intestinal IEC-6 cells. J Nutr, 2015,145(10): 2201-2206.
[25] 杨龙龙.miR-155在创面愈合中的作用及机制研究.西安: 第四军医大学,2015.
[26] MELNIK B C, JOHN S M, SCHMITZ G. Milk: an exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy?. J Transl Med, 2014,12: 43.
[27] BAIER S R, NGUYEN C, XIE F, et al. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J Nutr, 2014,144(10): 1495-1500.