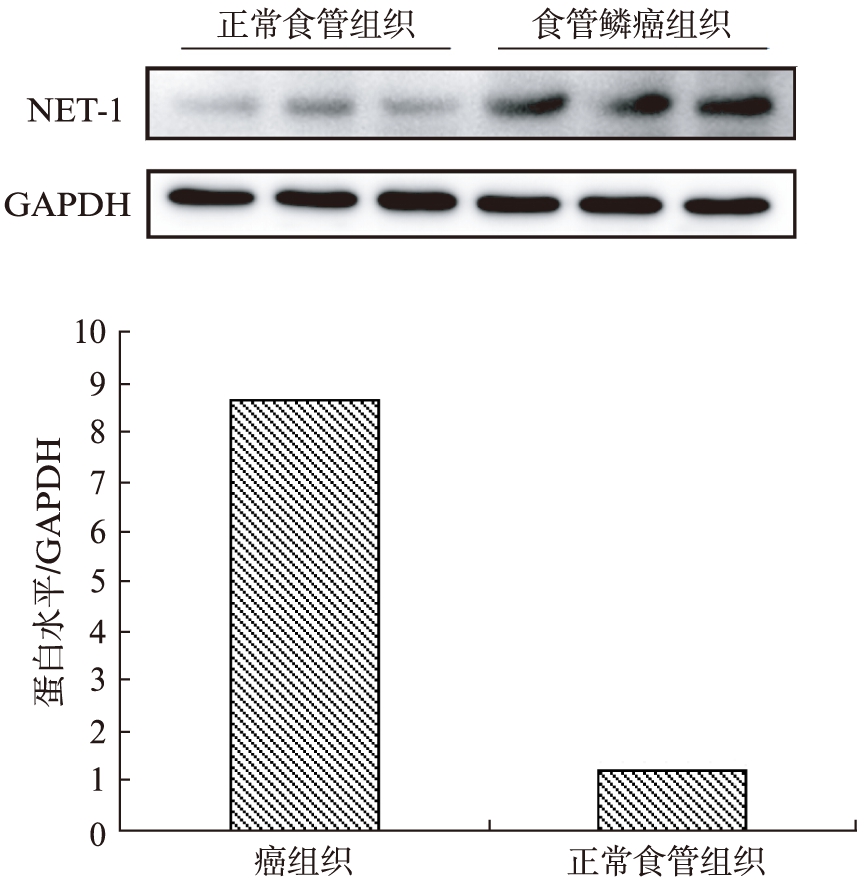
图1 ESCC组织及正常食管组织中NET1的表达情况
Fig.1 Expression of NET1 in ESCC and normal esophageal tissue
·基础研究·
【摘要】目的 探讨食管鳞状细胞癌(esophageal squamous cell carcinoma, ESCC)组织中神经上皮细胞转化基因1(neuroepithelial cell transforming gene 1, NET1)的表达及D2-40标记的肿瘤淋巴管浸润与病理之间的相关性。方法 采 用Western印迹法检测ESCC组织及正常食管组织中NET1蛋白的表达情况,免疫组织化学法检测ESCC中NET1及D2-40的表达情况,观察D2-40标记的淋巴管内癌细胞浸润(lymphatic vessel invasive, LVI)的情况,分析NET1、LVI与临床病理指标之间的关系及患者5年生存率的差异。结果 NET1在ESCC组织中阳性表达率(++/+++)为61.3%(20.4%/40.9%)(49/80),正常食管组织中表达阳性率为10.5%(2/19),2例均为弱阳性(+),LVI在ESCC组织中的阳性率为36.3%(29/80),NET1高表达、LVI阳性与ESCC的淋巴结阳性转移、临床分期呈正相关(P<0.05),与肿瘤的神经血管侵犯相关(P<0.05),而与性别、年龄、组织学分级无明显相关性(P>0.05)。NET1高表达与LVI阳性密切相关(P=0.012),NET1高表达或LVI阳性的ESCC患者术后的生存率明显降低,而且同时存在NET1高表达和LVI阳性的ESCC患者术后5年生存率最低。结论 联合检测NET1及LVI可以提高对食管鳞癌患者的术后临床病理分期及预后的判断的准确性。
【关键词】神经上皮细胞转化基因1; D2-40; 食管鳞状细胞肿瘤; 淋巴管浸润
淋巴结转移(lymph node metastasis, LNM)是食管鳞状细胞癌(esophageal squamous cell carcinoma, ESCC)最常见的转移方式,伴有LNM的ESCC患者预后较差[1- 5]。淋巴管浸润(lymphatic vessel invasion, LVI)是指在常规H-E染色切片中淋巴管内皮细胞围成的管腔内观察到肿瘤细胞,与LNM密切相关[6]。平足蛋白又称肾小球上皮细胞整合膜蛋白,是一种跨膜蛋白,在淋巴管内皮细胞上有表达,可作为淋巴管内皮的标志物[7]。D2-40可用于免疫组化检测平足蛋白,可客观地显示淋巴管[8]。NET1最初是从神经上皮瘤中分离鉴定出来的,与肿瘤的发生、发展、浸润及转移有关。NET1与ESCC的关系目前尚不清楚。
本研究收集ESCC标本,采用Western印迹法检测NET1表达情况,通过D2-40标记ESCC淋巴管,在H-E染色下观察并计数LVI,同时免疫组化检测NET1蛋白在ESCC中的表达情况,分析NET1的表达与LVI之间的相关性,通过生存分析NET1高表达、LVI阳性对ESCC患者术后生存率的影响。
1.1 材料
收集2010年1月至2012年12月南通市第一人民医院胸心血管外科ESCC标本80例,术前胃镜活检病理明确为鳞状细胞癌,均无术前化疗、放疗等治疗史。其中男性50例,女性30例,年龄46~78岁。术后病理: 组织学分级Ⅰ级27例,Ⅱ级32例,Ⅲ级21例;浸润黏膜及黏膜下层24例,侵达肌层28例,侵达浆膜28例;伴淋巴结转移60例,无淋巴结转移20例;TNM分期,Ⅰ期14例,Ⅱ期21例,Ⅲ期42例,Ⅳ期3例。全部标本分别在无坏死区癌灶、癌旁3cm内及远端正常食管取材。每例标本取材时均分为2份,1份取材后立即放置在液氮中冷冻,用于NET-1蛋白Western印迹法检测;另1份用福尔马林固定,用于NET-1蛋白免疫组化检测。
1.2 Western印迹法检测蛋白
提取食管鳞癌组织蛋白,置于-20℃保存,每个样品取20μl,加入等体积loading buffer,于聚丙烯酰胺凝胶电泳(5%积存胶,12%分离胶)分离蛋白,积存胶电压80V,分离胶电压120V,电泳时间约 1h。采用湿式转移法,在冰浴中350mA恒流转移2h;电转移后的PVDF膜用依次脱脂奶粉封闭,在 1∶ 200 NET-1一抗中室温孵育24h,1×TBST溶液洗膜,加1∶2000稀释的二抗,室温孵育2h,暗房中用ECL溶液显色,压片,显影,显示各个组织标本的NET-1蛋白表达情况,然后1×TBST溶液洗膜,在1∶ 400 β-actin一抗中4℃孵育24h,1×TBST溶液洗膜,加1∶2000稀释的二抗,室温孵育2h,暗房中用ECL溶液显色,压片,显影,显示内参蛋白表达情况。
1.3 免疫组化检测
按照试剂说明书检测NET-1表达。石蜡切片脱蜡至水,微波抗原修复,EnVision两步法过氧化物酶阻断液孵育5min,第一抗体NET-1(工作浓度1/200);室温30min,DAB显色。苏木精轻微衬染。NET-1阳性定位于胞浆或胞膜,呈红褐色颗粒状,阳性表达用半定量积分判断结果。阳性细胞≤5%为0分,6%~ 25%为1分,26%~50%为2分,51%~75%为3分,>75%为4分。阳性强度暗红为1分,红为2分,红褐色为3分。阳性细胞数与阳性强度相乘,0分为“-”,1~4分“+”,5~8分为“++”,9~ 12分为“+++”,++/+++为高表达[13]。
1.4 随访
随访截止日期为2015年12月31日,中位随访时间为42个月。对患者通过电话或门诊复查进行随访,记载患者的生存情况及在NET1不同表达情况及LVI阴性或阳性时总体5年的生存率差异。
1.5 统计学处理
应用SPSS 16.0行χ2检验及Pearson相关性分析,应用Kaplan-Meier法进行生存分析。P<0.05为差异有统计学意义。
2.1 ESCC及正常食管组织中NET1蛋白的表达情况
与正常的食管组织比较,ESCC组织中NET1蛋白表达水平明显升高,见图1。

图1 ESCC组织及正常食管组织中NET1的表达情况
Fig.1 Expression of NET1 in ESCC and normal esophageal tissue
2.2 食管鳞状细胞组织中NET1及D2-40的表达
NET1蛋白阳性信号主要分布于核周胞浆,为棕褐色颗粒,见图2。80例食管鳞癌患者中,49例(61.3%)NET1呈高表达(++/+++),正常食管组织中仅有10.5%的患者NET1呈阳性(+)表达(P<0.05)。D2-40阳性的淋巴管在食管鳞癌内及癌旁组织中均有表达,细胞浆染色呈棕黄色或褐色,呈管腔状或者条索状,管壁覆盖单层上皮细胞,管壁周围无清晰的平滑肌细胞和基底膜,管腔内无红细胞。LVI阳性表现为: D2-40阳性的淋巴管内可见单个、数个或成团的癌细胞。本组ESCC患者LVI阳性率为36.25%。

图2 食管鳞状细胞中NET1阳性蛋白表达
Fig.2 Expression of positive NET1 protein in esophageal squamous cell carcinomaA: NET1高表达;B: NET1低表达;C: LVI阳性;D: LVI阴性
2.3 NET1、LVI阳性与ESCC病理的关系
ESCC组织中NET1蛋白的表达与癌组织浸润深度、淋巴结转移、肿瘤分期有关(P<0.05),随着肿瘤浸润深度的增加、淋巴结的转移及肿瘤分期的增高,NET1高表达率也逐渐增高;而在不同性别、年龄、组织学分级患者中,NET1的表达差异无统计学意义,见表1。
2.4 NET1与LVI的相关性
23例患者NET1高表达和D2-40阳性,25例患者NET1低表达和D2-40阴性,NET1高表达与D2-40标记阳性LVI密切相关(r=0.280,P=0.012)。
2.5 NET1表达及LVI阴性或阳性对5年生存率的影响
NET1高表达的ESCC患者的5年生存率明显低于低表达的患者(P=0.002),见图3;D2-40标记LVI阳性的ESCC患者5年生存率低于阴性的患者(P<0.001),见图4;NET1高表达同时LVI阳性的ESCC患者5年生存率最低,NET1低表达同时LVI阴性的食管患者5年生存率较高(P<0.001),见图5。
表1 NET1的表达、D2-40标记的阳性LVI与ESCC组织临床病理资料的关系
Tab.1 The relationship of NET1 expressions and D2-40 labeled lymphatic vessel invasion with lymph node metastasis in esophageal squamous carcinoma

参数NNET1低表达(n=31)高表达(n=49)P值LVI(D2-40)阴性(n=51)阳性(n=29)P值年龄/岁0.610 ≥6039142522170.183 <604117242912性别0.167 男性5019310.8592921 女性301218228肿瘤位置0.8640.551 胸中上段21912129 胸中段281117208 胸下段3111201912手术方式0.4700.884 开放手术4519262916 胸腔镜手术3512232213pT分期<0.0010.013 Tis-124159213 T22812161612 T3283251414pN分期0.0050.022 阴性(N0)20137173 阳性(N1~3)6018423426pM分期0.5620.007 M07630465125 M141304血管浸润0.0190.017 阴性6830384721 阳性1211148神经浸润0.0250.007 阴性6529364619 阳性15213510分化程度0.8890.773 G183562 G2279181611 G33414202113 G4115683pTNM分期0.0680.074 ⅠA64251 ⅠB84471 ⅡA107382 ⅡB116583 ⅢA266201511 ⅢB1221039 ⅢC41331 Ⅳ31221
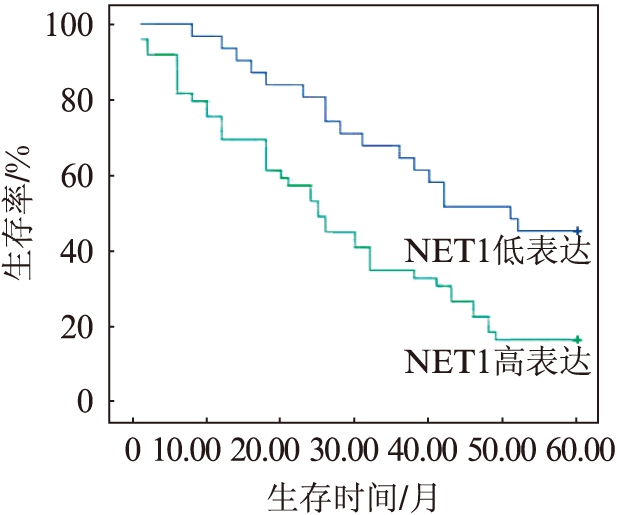
图3 NET1高表达与低表达患者5年生存曲线
Fig.3 Survival curve of 5-year in patients with NET1 high and low expression
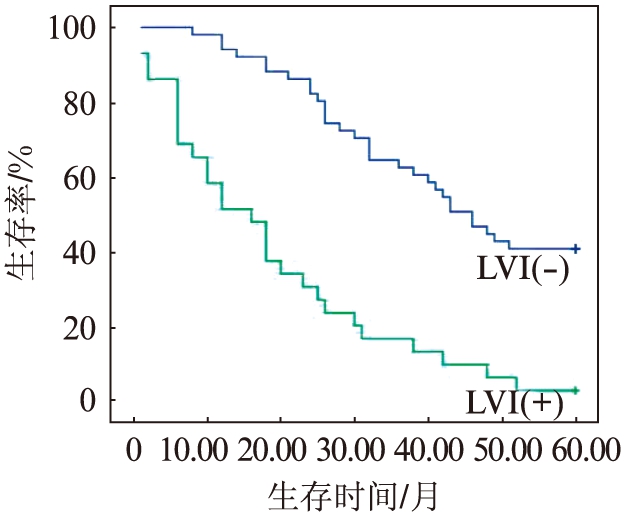
图4 LVI阴性与阳性患者5年生存曲线
Fig.4 Survival curve of 5-year in patients with LVI positive and negative
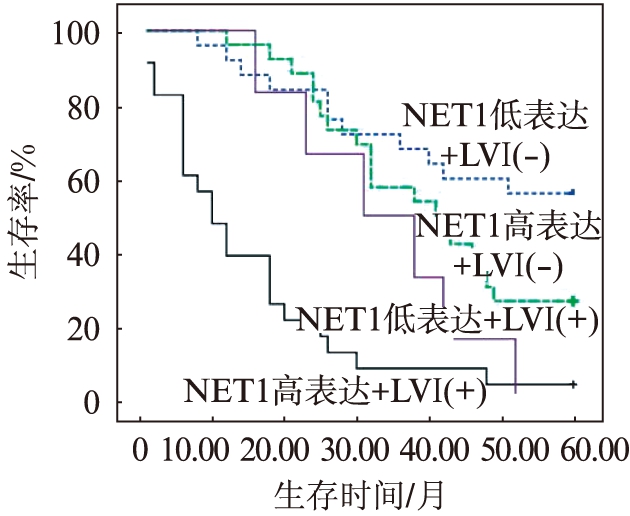
图5 NET1的表达与LVI阴性或阳性患者5年生存曲线
Fig.5 Survival curve of 5-year in patients with positive or negative NET1 expression and LVI
ESCC的发生、发展、转移与多种因素相关,其中ESCC细胞间黏附分子的改变导致细胞间黏附的缺失是其发生转移的关键步骤。NET1属于鸟核苷酸交换因子家族成员,能够帮助小G蛋白GDP和GTP间的相互转化,因而NET1可以特异性激活Rho家族蛋白RhoA,其主要的生物学效应是调控肌动蛋白细胞骨架的聚集和重构、细胞间黏附的分离和细胞基质的分解重构,与癌细胞的转移密切相关[9]。淋巴结转移是判断ESCC患者预后不良的一个重要预测因子。通过D2-40标记淋巴管检测到淋巴管浸润表明淋巴结转移已突破第1步。本研究通过D2-40标记ESCC旁组织中淋巴管平足蛋白的表达检测淋巴管浸润的情况,分析淋巴管浸润与NET1表达及ESCC临床病理之间的关系。
NET1在多种肿瘤中高表达,参与肿瘤的发生、发展、浸润及转移[9-14]。研究[13],发现NET1与非小细胞肺癌的生物学行为密切相关,但NET1与ESCC的关系尚不明确。本研究结果初步揭示了NET1与ESCC临床病理的相关性。本组病例中NET1的高表达率达61.3%,与ESCC的临床病理分期密切相关,随着ESCC浸润深度的增加,NET1高表达率也不断增加(Tis-1 37.5%、 T2 57.1%、 T3 89.3%, P<0.001),伴有淋巴结转移ESCC患者的NET1高表达率(N1-3, 70%)明显高于无淋巴结转移(N0,35%)的ESCC患者(P= 0.005)。伴有血管或神经侵犯的ESCC患者的NET1高表达率明显增加(P=0.019、P=0.025)。
本研究通过D2-40标记淋巴管,检测LVI的阳性率为36.52%,与文献[15-16]报道数据(33%~89%)相比处于偏低的阳性率,其差异的原因主要与入组患者的差异性有关,与LVI计数的标准有一定关系,本组对于可疑阳性的标本均予排除。在LVI与ESCC临床病理关系的研究中发现: 淋巴结阳性转移的患者中LVI阳性率达到43.3%,本组20例淋巴结转移阴性的ESCC患者中有3例患者出现LVI阳性,虽然他们的T分期为Tis-1,这表明部分早期的ESCC患者已伴有淋巴管的扩散,LVI的检测对于这部分患者的预后判断尤为重要。
本研究结果提示,NET1高表达与D2-40标记的LVI阳性密切相关。ESCC通过淋巴管到淋巴结转移是非常重要的转移途径。LVI阳性提示着ESCC细胞已经侵入淋巴管,表明该ESCC患者的癌细胞开始向远处转移,部分患者未达到淋巴结(淋巴结检测阴性)或部分患者癌细胞已达远处淋巴结(区域淋巴结检测为阳性)。NET1高表达的ESCC患者容易出现LVI阳性,这正与上述的NET1生物特性密切相关。因此,联合检测NET1的表达和LVI阳性与否,提高ESCC患者病理学分期的准确性,对患者后期的治疗具有指导性价值。
联合检测NET1的表达和LVI阳性与否对患者预后的判断也有非常重要的意义。本研究发现: NET1高表达或LVI阳性的ESCC患者术后的生存率明显降低,而且同时存在NET1高表达和LVI阳性的ESCC患者术后5年生存率最低。因此,ESCC患者若同时出现NET1高表达和LVI阳性提示预后不良。
本组标本检测时选取肿瘤中心部位组织及癌旁组织,无法对所有患者的ESCC全部标本进行检测,可能存在部分实际LVI阳性的患者未能统计到。还需要投入更多人力财力完善标本的检测。目前,研究[17-18]在肺癌患者的外周血中发现含有肺癌相关信息物质的外泌体(exosome),进一步的研究将采集大样本食管患者的外周血提取exosome,检测NET1的转录及表达情况,然后通过细胞及动物水平评价NET1在ESCC中的作用。
【参考文献】
[1] Eguchi T, Nakanishi Y, Shimoda T, et al. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases[J]. Mod Pathol, 2006,19: 475-480.
[2] Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan[J]. Surgery, 1998,123: 432-439.
[3] Shiozaki H, Doki Y, Yamana H, et al. A multi-institutional study of immunohistochemical investigation for the roles of cyclin D1 and E-cadherin in superficial squamous cell carcinoma of the esophagus[J]. J Surg Oncol, 2002,79: 166-173.
[4] Imamura Y, Hayashi N, Sato N, et al. Extensive lymphatic spread of cancer cells in patients with thoracic esophageal squamous cell carcinoma: detection of CEA-mRNA in the three field lymph nodes[J]. J Surg Oncol, 2010,102: 509-515.
[5] Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study-JCOG9204[J]. J Clin Oncol, 2003,21: 4592-4596.
[6] Ishii M, Ota M, Saito S, et al. Lymphatic vessel invasion detected by monoclonal antibody D2-40 as a predictor of lymph node metastasis in T1 colorectal cancer[J]. Int J Colorectal Dis, 2009,24: 1069-1074.
[7] Schacht V, Dadras SS, Johnson LA, et al. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors[J]. Am J Pathol, 2005,166: 913-921.
[8] Gockel I, Domeyer M, Sgourakis GG, et al. Prediction model of lymph node metastasis in superficial esophageal adenocarcinoma and squamous cell cancer including D2-40 immunostaining[J]. J Surg Oncol, 2009,100: 191-198.
[9] Carr HS, Zuo Y, Oh W, et al. Regulation of focal adhesion kinase activation, breast cancer cell motility, and amoeboid invasion by the RhoA guanine nucleotide exchange factor Net1[J]. Mol Cell Biol, 2013,33: 2773-2786.
[10] Kawata H, Shimada N, Kamiakito T, et al. RhoC and guanine nucleotide exchange factor Net1 in androgen-unresponsive mouse mammary carcinoma SC-4 cells and human prostate cancer after short-term endocrine therapy[J]. Prostate, 2012,72: 1071-1079.
[11] He S, Wei YZ, Wang GL, et al. Study of RNA interference targeting NET-1 combination with sorafenib for hepatocellular carcinoma therapy in vitro and in vivo[J]. Gastroenterol Res Pract, 2013,2013: 685150.
[12] Zhang J, Wang J, Chen L, et al. Expression and function of NET-1 in human skin squamous cell carcinoma[J]. Arch Dermatol Res, 2014,306: 385-397.
[13] Huang HT, Qiao Y, Chen L, et al. Dual silencing of NET-1 and VEGF genes affect biological behavior of lung cancer in vitro experiment[J]. Acta Medica Mediterr, 2015,31(1): 73-81.
[14] Ji ZJ, Wang JL, Chen L. Inhibition of skin squamous cell carcinoma proliferation and promote apoptosis by dual silencing of NET-1 and survivin[J]. Oncol Rep, 2015,34: 811-822.
[15] Xue L, Lu HQ, He J, et al. Expression of FOXP3 in esophageal squamous cell carcinoma relating to the clinical data[J]. Dis Esophagus, 2010,23: 340-346.
[16] Shimada H, Okazumi S, Matsubara H, et al. Impact of the number and extent of positive lymph nodes in 200 patients with thoracic esophageal squamous cell carcinoma after three-field lymph node dissection[J]. World J Surg, 2006,30: 1441-1449.
[17] Qin X, Xu H, Gong W, et al. The tumor cytosol miRNAs, fluid miRNAs, and exosome miRNAs in lung cancer[J]. Front Oncol, 2015,4: 357.
[18] Li J, Jun Yu, Liu A, et al. β-Elemene against human lung cancer via up-regulation of P53 protein expression to promote the release of exosome[J]. Lung Cancer, 2014,86: 144-150.
Relationship of NET1 expression and D2- 40 labeled lymphatic vessel invasion with clinicopathologic features of esophageal squamous cell carcinoma
【Abstract】Objective To study the relationship of NET1 expression and D2-40 labeled lymphatic vessel invasion(LVI) with clinicopathological characteristics of esophageal squamous cel carcinoma(ESCC). Methods The expression of NET1 in ESCC and normal esophageal tissue was detected by Western blot. Immunohistochemistry was used to detect the expression of NET1 and D2-40 protein in 80 cases of ESCC and D2-40 labeled LVI was identified. The relationship of NET1 expressions and D2-40 labeled LVI with clinical pathological characteristics of ESCC was analyzed. The overall 5-year survival rate was analyzed by Kaplan-Meier survival analysis. Results The positive expression(++/+++) rate of NET1 was 61.3%(20.4%/40.9%) in ESCC and 10.5%(+) in normal esophageal tissue. NET1 high expression and positive LVI was significantly correlated with positive lymph node metastasis, TNM stage, nerve and blood vessel invasion(P<0.05), but not with gender, age, tumor differentiation(P>0.05). The positive correlation between NET1 high expression and positive LVI was found(P=0.012). The 5-year survival rate of patients with NET1 high expression or positive LVI was significantly lower. The 5-year survival rate of patients with NET1 high expression and positive LVI was lowest. Conclusion Combined detection of NET1 and D2-40 is of value in clinical staging and prognosis of esophageal squamous cell carcinoma.
【Key words】neuroepithelial cell transforming gene 1; D2-40; esophageal squamous cell carcinoma; lymphatic vessel invasive
doi:10.16118/j.1008-0392.2016.05.007
收稿日期:2016-06-04
基金项目:南通市卫生局青年基金(WQ2014020)
【中图分类号】R 735.1
【文献标志码】A
【文章编号】1008-0392(2016)05-0034-06